This is an old revision of this page, as edited by Beetstra (talk | contribs) at 09:26, 6 December 2011 (Saving copy of the {{chembox}} taken from revid 464263360 of page Sodium_bicarbonate for the Chem/Drugbox validation project (updated: '').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:26, 6 December 2011 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 464263360 of page Sodium_bicarbonate for the Chem/Drugbox validation project (updated: '').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 464263360 of page Sodium_bicarbonate with values updated to verified values. |
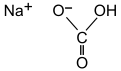
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sodium hydrogen carbonate | |||
| Other names Baking soda, bicarbonate of soda, nahcolite, sodium bicarbonate, sodium hydrogencarbonate | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 4153970 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| KEGG | |||
| MeSH | Sodium+bicarbonate | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | CHNaO3 | ||
| Molar mass | 84.006 g·mol | ||
| Appearance | White crystals | ||
| Density | 2.20 g cm | ||
| Melting point | 50 °C (122 °F; 323 K) | ||
| Boiling point | 851 °C (1,564 °F; 1,124 K) | ||
| Solubility in water | 9 g/100 mL
69 g/L (0 °C) | ||
| log P | -0.82 | ||
| Acidity (pKa) | 10.329
6.351 (carbonic acid) | ||
| Refractive index (nD) | 1.3344 | ||
| Pharmacology | |||
| Routes of administration |
Intravenous, oral | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Causes serious eye irritation | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 4.22 g kg | ||
| Related compounds | |||
| Other anions | Sodium carbonate | ||
| Other cations | Ammonium bicarbonate | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- "Physical Constants of Inorganic Compounds". CRC Handbook, p. 4-85.
- ^ "Aqueous solubility of inorganic compounds at various temperatures". CRC Handbook, p. 8-116.
- ^ "Sodium Bicarbonate" (PDF). UNEP Publications.
- ^ Goldberg, Robert N.; Kishore, Nand; Lennen, Rebecca M. "Thermodynamic quantities for the ionization reactions of buffers in water". CRC Handbook. pp. 7–13.

