This is an old revision of this page, as edited by Nrcprm2026 (talk | contribs) at 22:34, 18 April 2006 (→bond valence parameters: the estimates in this section contradict experimentally-derived values; blanking). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 22:34, 18 April 2006 by Nrcprm2026 (talk | contribs) (→bond valence parameters: the estimates in this section contradict experimentally-derived values; blanking)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| Uranium trioxide | |
|---|---|

|
solid γ-UO3 (gamma polymorph) oxygen diameters sharply reduced for visibility |
| General | |
| Systematic name | Uranium trioxide Uranium(VI) oxide |
| Other names | Uranyl oxide Uranic oxide |
| Molecular formula | UO3 (or O3U) |
| CAS number | |
| Properties | |
| Molar mass | 286.2873 g/mol Commercial samples may have undergone isotope fractionation, and their molecular mass may be significantly different |
| Density and phase | 5.5 – 8.7 g/cm |
| Solubility (water) | Partially soluble |
| Solubility (dog lung fluid) | < 5 days (Morrow, 1972) |
| Melting point | ~ 200 – 650 °C decomp. (s) |
| Structure | |
| Molecular shape | T-shape |
| Coordination geometry |
γ-UO3: |
| Crystal structure | I41/amd (γ-UO3) |
| Hazards | |
| MSDS | UO3-MSDS |
| Main hazards | highly toxic: teratogen, immunotoxin, neurotoxin, genotoxin, nephrotoxin |
| Flash point | inflamable |
| Related compounds | |
| Other anions | Uranyl nitrate |
| Other cations | Chromium trioxide |
| Related compounds | Uranium dioxide Triuranium octaoxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The toxic, teratogenic, and radioactive solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.
UO3 gas is produced at high temperatures from U3O8, which comprises 75% of the particulate combustion product of uranium burning in air. The partial pressure of UO3(g) is several dozen mbar well below uranium's burning temperature. Health impact assessments for depleted uranium munitions should take into account the presence of respiratory UO3.
Production and use
There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium entrichment.
- U3O8 can be oxidized at 500°C with oxygen.
- Uranyl nitrate, (UO2(NO3)2·6H2O) can be heated to yield UO3. This occurs during the reprocessing of nuclear fuel. Fuel rods are dissolved in HNO3 to separate uranyl nitrate from plutonium and the fission products (the PUREX method). The pure uranyl nitrate is converted to solid UO3 by heating at 400 °C. After reduction with hydrogen (with with other inert gas present) to uranium dioxide, the uranium can be used in new MOX fuel rods.
- Ammonium diuranate or sodium diuranate (Na2U2O7·6H2O) may be decomposed. Sodium diuranate, also known as yellowcake, is converted to uranium trioxide in the enrichment of uranium. Uranium dioxide and uranium tetrafluoride are intermediates in the process which ends in uranium hexafluoride.
Uranium trioxide is shipped between processing facilities in the form of a gel.
Cameco Corporation, which operates at the world's largest uranium refinery at Blind River, Ontario, produces high-purity uranium trioxide.
Health and safety hazards
Like all hexavalent uranium compounds (also called uranium(VI) compounds), UO3 is hazardous by inhalation, ingestion, and through skin contact. It is a poisonous, radioactive substance, which may cause shortness of breath, coughing, acute arterial lesions, and changes in the chromosomes of white blood cells and gonads leading to congenital malformations if inhaled.
Chemistry and structure
Solid state
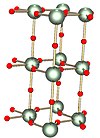

The only well characterized binary trioxide of any actinide is UO3, of which several polymorphs are known. Solid UO3 loses O2 on heating to give green-colored U3O8: reports of the decomposition temperature in air vary from 200–650 °C. Heating at 700 °C under H2 gives dark brown uranium dioxide (UO2), which is used in MOX nuclear fuel rods.
Uranium trioxide reacts at 400 °C with freon-12 to form chlorine, phosgene, carbon dioxide and uranium(IV) fluoride. The freon-12 can be replaced with freon-11 which forms carbon tetrachloride instead of carbon dioxide. This is a case of a hard perhalogenated freon which is normally considered to be inert being converted chemically at a moderate temperature.
2 CF2Cl2 + UO3 → UF4 + CO2 + COCl2 + Cl2
4 CF2Cl2 + UO3 → UF4 + 3COCl2 + CCl4 + Cl2
Uranium trioxide can be dissolved in a mixture of tributyl phosphate and thenoyltrifluoroacetone in supercritical carbon dioxide, ultrasound was employed during the dissolution.
The most frequently encountered polymorph is γ-UO3, whose x-ray structure has been solved from powder diffraction data. The compound crystallizes in the space group I41/amd with two uranium atoms in the asymmetric unit. Both are surrounded by somewhat distorted octahedra of oxygen atoms. One uranium atom has two closer and four more distant oxygen atoms whereas the other has four close and two more distant oxygen atoms as neighbors. Thus it is not incorrect to describe the structure as , that is uranyl uranate. .
High presure solid forms exist. Gmelin Handbuch (1982) U-C1, 129-135.
-
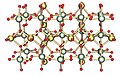 The γ (gamma) form, with the different uranium environments in green and yellow
The γ (gamma) form, with the different uranium environments in green and yellow
-
 The environment of the uranium atoms shown as yellow in the gamma form
The environment of the uranium atoms shown as yellow in the gamma form
-
 The chains of U2O2 rings in the gamma form in layers, alternate layers running at 90 degrees to each other. These chains are shown as containing the yellow uranium atoms, in a octahedral environment which are distorted towards square planar by an elongation of the axial oxygen-uranium bonds.
The chains of U2O2 rings in the gamma form in layers, alternate layers running at 90 degrees to each other. These chains are shown as containing the yellow uranium atoms, in a octahedral environment which are distorted towards square planar by an elongation of the axial oxygen-uranium bonds.
-
 The delta (δ) form was reported by Weller et al. (1988) Polyhedron, 7, 243-244.
The delta (δ) form was reported by Weller et al. (1988) Polyhedron, 7, 243-244.
Gas phase
Infrared spectroscopy of molecular UO3 isolated in an argon matrix indicates a T-shaped structure (point group C2v) for the molecule. This is in contrast to the commonly encountered D3h symmetry exhibited by most trioxides. From the force constants the authors deduct the U-O bond lengths to be between 1.76 and 1.79 angstroms (176 to 179 picometers).
At elevated temperatures gaseous UO3 and O2 are in equilibrium with solid U3O8.
- /3 U3O8(s) + /6 O2(g) UO3(g)
With increasing temperature the equilibrium is shifted to the right. This system has been studied at temperatures between 900 and 1500 °C. The vapor pressure of monomeric UO3 is low but appreciable, about 10 mbar (1 mPa) at 980 °C, rising to 10 mbar (10 Pa) at 1400 °C, 0.34 mbar (34 Pa) at 1800 K, 19 mbar (1.9 kPa) at 2000 K, and 81 mbar (8.1 kPa) at 2200 K. The burning temperature of uranium in air usually exceeds 2500 K.
Aerial oxidation of any uranium compound eventually results in the formation of a uranyl compound, and uranium trioxide is the only uranyl oxide.
Uranium oxides in ceramics
UO3-based ceramics become green or black when fired in a reducing atmosphere and yellow to orange when fired with oxygen. Orange-coloured Fiestaware is a well-known example of a product with a uranium-based glaze. UO3-has also been used in formulations of enamel, uranium glass, and porcelain.
Prior to 1960, UO3 was used as an agent of crystallization in crystalline coloured glazes. It is possible to determine with a Geiger counter if a glaze or glass was made from UO3.
Related anions and cations
Uranium oxide is amphoteric and reacts as acid and as a base, depending on the conditions.
As an acid:
- UO3 + H2O → UO4 + H
Dissolving uranium oxide in a strong base like sodium hydroxide forms the doubly negatively charged uranate anion (UO4). Uranates tend to agglomerate, forming diuranate, U2O7
As a base:
- UO3 + H2O → UO2 + OH
Dissolving uranium oxide in a strong acid like sulfuric or nitric acid forms the double positive charged uranyl cation. The uranyl nitrate formed (UO2(NO3)2ˑ6H2O) is soluble in ethers, alcohols, ketones and esters; for example, tributylphosphate. This solubilty is used to separate uranium from other elements in nuclear reprocessing, which begins with the dissolution of nuclear fuel rods in nitric acid. The uranyl nitrate is then converted to uranium trioxide by heating.
From nitric acid one obtains uranyl nitrate, trans-UO2(NO3)2·2H2O, consisting of eight-coordinated uranium with two bidentate nitrato ligands and two water ligands as well as the familiar O=U=O core.
References
- Sheft I, Fried S, Davidson N (1950). "Preparation of Uranium Trioxide". Journal of the American Chemical Society. 72: 2172–2173.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI] - Dell RM, Wheeler V J (1962). "Chemical Reactivity of Uranium Trioxide Part 1.conversion to U3O8, U02 and UF4". Transaction Faraday Society: 1590–1607. DOI
- Morrow, PE, Gibb FR, Beiter HD (1972). "Inhalation studies of uranium trioxide". Health Physics. 23: 273–280.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Abstract - Sutton M, Burastero SR (2004). "Uranium(VI) solubility and speciation in simulated elemental human biological fluids". Chemical Research in Toxicology. 17: 1468–1480. DOI
- Trofimov TI, Samsonov MD, Lee SC, Myasoedov BF, Wai CM (2001). "Dissolution of uranium oxides in supercritical carbon dioxide containing tri-n-butyl phosphate and thenoyltrifluoroacetone". Mendeleev Communications. 11: 129–130.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Booth HS, Krasny-Ergen W,Heath RE (1946). "Uranium Tetrafluoride". Journal of the American Chemical Society. 68: 1969–1970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Engmann R, de Wolff PM (1963). "The Crystal Structure of γ-UO3". Acta Crystallographica. 16: 993. DOI
- Wilson WB (1961). "High-Pressure High-Temperature Investigation of the Uranium-Oxygen System". Journal Inorganic Nuclear Chemistry. 19: 212–222. DOI
- Gabelnick SD, Reedy GT, Chasanov MG (1973). "Infrared spectra of matrix-isolated uranium oxide species. II: Spectral interpretation and structure of UO3". 59: 6397.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - Ackermann RJ, Thorn RJ, Alexander C, Tetenbaum M (1960). "Free Energies of Formation of Gaseous Uranium, Molybdenum, and Tungsten Trioxides". Journal of Physical Chemistry. 64: 350–355.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI page350 351 352 353 354 355 - Ackermann RJ, Gilles PW, Thorn RJ (1956). Journal of Chemical Physics. 25: 1089.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - Alexander CA (2005). "Volatilization of urania under strongly oxidizing conditions". Journal of Nuclear Materials. 346: 312–318. DOI
- Mouradian and Baker (1963). "Burning Temperatures of Uranium and Zirconium in Air". Nuclear Science and Engineering. 15: 388–394.
- Gilchrist RL, Glissmyer JA, Mishima J (1979). "Characterization of Airborne Uranium from Test Firings of XM774 Ammunition," Technical report no. PNL-2944 Richland, WA: Battelle Pacific Northwest Laboratory".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - Cotton, S (1991). Lanthanides and Actinides. New York.
{{cite book}}: Text "page 128" ignored (help)CS1 maint: location missing publisher (link) - Stuart (1979). "Solubility and Hemolytic Activity of Uranium Trioxide". Environmental Research. 18: 385–396. DOI
- Neumüller O-A (1988). Römpps Chemie-Lexikon (6 ed.). Stuttgart: Frankh'sche Verlagshandlung. ISBN 3-440-04516-1.
- Gmelin Handbook of Inorganic Chemistry (8 ed.). 1977.
{{cite book}}: Text "page 98" ignored (help) - Gmelin Handbuch der anorganischen Chemie (8 ed.). 1977.
{{cite book}}: Text "page 118-120" ignored (help) DOI
- Green, DW (1980). "Relationship between spectroscopic data and thermodynamic functions; application to uranium, plutonium, and thorium oxide vapor species". Journal of Nuclear Materials. 88: 51–63. DOI
- Salbu, B. et al. (2005) "Oxidation states of uranium in depleted uranium particles from Kuwait," Journal of Environmental Radioactivity, 78, 125–135.
- Nakajima K, Arai Y (2001). "Mass-spectrometric investigation of) UO3 (g)". J Nuclear Mater. 294: 250–255.
- Ackermann RJ, Gilles PW, Thorn RJ (1956). "High-Temperature Thermodynamic Properties of Uranium Dioxide". J Chem Phys. 25: 1089–1097.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Berkowitz J, Inghram MG, Chupka WA . (1957). "Polymeric Gaseous Species in the Sublimation of Molybdenum Trioxide". J Chem Phys. 26: 842.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Ackermann RJ, Chang AT (1973). Thermodynamic Characterization of U3O8-z Phase (5 ed.).
{{cite book}}: Text "page 873-890" ignored (help) - Chapman AT, Meadows RE (1964). "Volatility of UO2+/-x and Phase Relations in the System Uranium Oxygen". J Am Ceramic Soc. 47: 614–621.
- Drowart J, Pattoret A, Smoes S (1964). "Heat of sublimation of uranium and consistency of thermodynamic data for uranium compounds". J Nuclear Materials. 12: 319–322.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Hoekstra HR, Siegel (1970). "Uranium-Oxygen System at high pressure". J Inorg Nuclear Chem. 32: 3237–3248.
- Hutchings, G. J. (1996). "Uranium-Oxide-Based Catalysts for the Destruction of Volatile Chloro-Organic compounds". Nature. 384: 341–343.
- Chatillion C, Defoort F, Froment K (2005). "Mass spectrometric critical assessment of thermodynamic data for UO3 (g)". J Phys Chem Solid. 66: 379–382.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Roberts LEJ, Walter AJ (1961). "Equilibrium Pressures and Phase Relations in the Uranium Oxide System". Journal of Inorganic and Nuclear Chemistry. 22: 213–229.
- Rauh EG, Ackermann RJ (1974). "First ionization potentials of some refractory oxide vapors". J Chem Phys. 60: 1396.
- Cort B (1987). "Infrared Characterization of Uranium Oxide Powders Using a Metal Light Pipe". Applied Spectroscopy. 41: 493–495.
- Chazel V (1998). "Effect of U3O8 specific surface area on in vitro dissolution, biokinetics, and dose coefficients". Radiation Protection Dosimetry. 79: 39–42.
- Ansoborlo E (1998). "Exposure implications for uranium aerosols formed at a new laser enrichment facility: application of the ICRP respiratory tract and systemic model". Radiation Protection Dosimetry. 79: 23–27. Abstract: "... urine assay could be useful, provided that measurements are made soon after a known acute intake."
- Stradling GN (2000). "Treatment for Actinide-bearing Industrial Dusts and Aerosols". Radiation Protection Dosimetry. 87: 41–50. Abstract
- Chazel V (2000). "Variation of solubility, biokinetics and dose coefficient of industrial uranium oxides according to specific surface area". Radiation Protection Dosimetry. 88: 223–231.
- Ansoborlo E (2002). "Determination of the physical and chemical properties, biokinetics, and dose coefficients of uranium compounds handled during nuclear fuel fabrication in France". Health Physics. 82: 279–289.
- Stradling N (2003). "Optimising monitoring regimens for inhaled uranium oxides". Radiation Protection Dosimetry. 105: 109–114.
- Stradling N (2003). "Anomalies between radiological and chemical limits for uranium after inhalation by workers and the public". Radiation Protection Dosimetry. 105: 175–178.
- Khaskelis, A.I (2004). "Uranium oxide weathering: spectroscopy and kinetics". Transactions of the American Nuclear Society. 91: 890–891. Abstract: "During nuclear fuel fabrication or reprocessing stages of a nuclear fuel cycle, it is possible for small particles of uranium oxides to escape into the environment. This paper reports measurements of rates of oxidation of uranium dioxide particles in controlled gas environments using in-situ phosphorescence spectroscopy. Comparison is made with reduction and reoxidation of uranium trioxide...." From Schueneman
- Schuenemana RA, Khaskelisa AI, Eastwooda D, van Ooijb WJ, Burggraf LW (2003). "Uranium oxide weathering: spectroscopy and kinetics". Journal of Nuclear Materials. 323: 8–17.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Chatillon, C (2005). "Mass spectrometric critical assessment of thermodynamic data for UO3(g)". Journal of Physics and Chemistry of Solids. 66: 379–383. DOI
- Busby C, Morgan S. "Did the use of Uranium weapons in Gulf War 2 result in contamination of Europe?". European Biology and Bioelectromagnetics. 1(5): 650–668.
- Hoekstra HR, Siegel S (1958). "Recent Developments in the Chemistry of the Uranium-Oxygen System". Proceedings of the Second International Conference on Peaceful Uses of Atomic Energy (Geneva: UN). 7: 394–400.
- H. Wanner and I. Forest, eds. (2004) Chemical Termodynamics of Uranium (Paris: OECD and French Nuclear Energy Agency)
- URANIUM and CERAMICS by Edouard Bastarache
- Uranium and Insoluble Compounds from the Occupational Safety and Health Administration; note that UO3 is moderately soluble (Morrow, 1972.)
- Chemical data from NIST
 UO3(g)
UO3(g)