This is an old revision of this page, as edited by Andrew73 (talk | contribs) at 16:05, 14 December 2005 (→References: added Saad reference). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 16:05, 14 December 2005 by Andrew73 (talk | contribs) (→References: added Saad reference)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)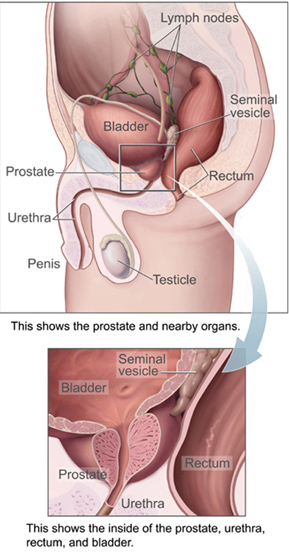
Prostate cancer is a disease in which cancer develops in the prostate, a gland in the male reproductive system. Cancer occurs when cells of the prostate mutate and begin to multiply out of control. These cells may spread (metastasize) from the prostate to other parts of the body, especially the bones and lymph nodes. Prostate cancer can cause pain, difficulty urinating, erectile dysfunction, and other symptoms.
Prostate cancer only occurs in men and develops most frequently in individuals over fifty years old. It is the second most common type of cancer in men and is responsible for more deaths than any cancer except for lung cancer. However, many men who develop prostate cancer never have symptoms, undergo no therapy, and eventually die of other causes. Many factors, including genetics and diet, have been implicated in the development of prostate cancer, but as of 2005, it is not a preventable disease.
Prostate cancer is most often discovered by screening blood tests, such as the PSA (prostate specific antigen) test or by physical examination of the prostate gland by a health care provider. Suspected prostate cancer is typically confirmed by removing a piece of the prostate (biopsy) and examining it under a microscope. Further tests, such as X-rays and bone scans, may be performed to determine whether prostate cancer has spread.
Prostate cancer can be treated with surgery, radiation therapy, hormone therapy, occasionally chemotherapy, or some combination of these. The age and underlying health of the man as well as the extent of spread, appearance under the microscope, and response of the cancer to initial treatment are important in determining the outcome of the disease. Since prostate cancer is a disease of older men, many men will die of other causes before the prostate cancer can spread or cause symptoms. This makes treatment selection difficult.
Medical condition| Prostate cancer | |
|---|---|
| Specialty | Oncology, urology |
The prostate
Main article: prostateThe prostate is a male reproductive organ which helps make and store semen. In adult men a typical prostate is about three centimeters long and weighs about twenty grams. It is located in the pelvis, under the urinary bladder and in front of the rectum. The prostate surrounds part of the urethra, the tube that carries urine from the bladder during urination and semen during ejaculation. Because of its location, prostate diseases often affect urination, ejaculation, or defecation. The prostate contains many small glands which make about twenty percent of the fluid comprising semen. In prostate cancer the cells of these prostate glands mutate into cancer cells. The prostate glands require male hormones, known as androgens, to work properly. Androgens include testosterone, which is made in the testes; dehydroepiandrosterone, made in the adrenal glands; and dihydrotestosterone, made in the prostate itself. Androgens are also responsible for secondary sex characteristics such as facial hair and increased muscle mass.
Symptoms
Early prostate cancer usually causes no symptoms. Often it is diagnosed during the workup for an elevated PSA noticed during a routine checkup. Sometimes, however, prostate cancer does cause symptoms, often similar to those of diseases such as benign prostatic hypertrophy. These include frequent urination, increased urination at night, difficulty starting and maintaining a steady stream of urine, blood in the urine, and painful urination. Prostate cancer may also cause problems with sexual function, such as difficulty achieving erection or painful ejaculation. Advanced prostate cancer may cause additional symptoms as the disease spreads to other parts of the body. The most common symptom is bone pain, often in the vertebrae (bones of the spine), pelvis or ribs, from cancer which has spread to these bones. Prostate cancer in the spine can also compress the spinal cord, causing leg weakness, urinary and fecal incontinence.
Pathophysiology
Prostate cancer is an adenocarcinoma, or glandular cancer, that begins when normal semen-secreting prostate gland cells mutate into cancer cells. Initially, small clumps of cancer cells remain confined to otherwise normal prostate glands, a condition known as carcinoma in situ or prostatic intraepithelial neoplasia. Over time these cancer cells begin to multiply and spread to the surrounding prostate tissue (the stroma) forming a tumor. Eventually, the tumor may grow large enough to invade nearby organs such as the seminal vesicles or the rectum, or the tumor cells may develop the ability to travel in the bloodstream and lymphatic system. Prostate cancer is considered a malignant tumor because it is a mass of cells which can invade other parts of the body. This invasion of other organs is called metastasis. Prostate cancer most commonly metastasizes to the lymph nodes, the rectum, the bladder, and the bones.
Epidemiology
A man's risk of developing prostate cancer is related to his age, genetics, race, diet, lifestyle, medications, and other factors. The primary risk factor is age. Prostate cancer is uncommon in men less than 45, but becomes more common with advancing age. The average age at the time of diagnosis is 70. However, many men never know they have prostate cancer. Autopsy studies of men who died of other causes have found prostate cancer in thirty percent of men in their 50s, and in eighty percent of men in their 70s. In the year 2005 in the United States, there were an estimated 230,000 new cases of prostate cancer and 30,000 deaths due to prostate cancer.
A man's genetic background contributes to his risk of developing prostate cancer. This is suggested by an increased incidence of prostate cancer found in certain racial groups, in identical twins of men with prostate cancer, and in men with certain genes. In the United States, prostate cancer more commonly affects black men than white or Hispanic men, and is also more deadly in black men. Men who have a brother or father with prostate cancer have twice the usual risk of developing prostate cancer. Studies of twins in Scandinavia suggest that forty percent of prostate cancer risk can be explained by inherited factors. However, no single gene is responsible for prostate cancer; many different genes have been implicated. Two genes (BRCA1 and BRCA2) that are important risk factors for ovarian cancer and breast cancer in women have also been implicated in prostate cancer.
Dietary amounts of certain foods, vitamins, and minerals can contribute to prostate cancer risk. Higher consumption of animal fat and lower intake of fruits and vegetables both increase prostate cancer risk. These effects are probably related to increased intake of alpha-linoleic acid (found in animal fat) at the expense of linoleic acid (found in vegetable oils). Other dietary factors that may increase prostate cancer risk include lower intake of vitamin E (found in green, leafy vegetables), lycopene (found in tomatoes), omega-3 fatty acids (found in fatty fishes like salmon), and the mineral selenium. Lower blood levels of vitamin D also may increase the risk of developing prostate cancer. This may be linked to lower exposure to ultraviolet (UV) light, since UV light exposure can increase vitamin D in the body.
There are also some links between prostate cancer and medications, medical procedures, and medical conditions. Daily use of anti-inflammatory medicines such as aspirin, ibuprofen, or naproxen may decrease prostate cancer risk. Use of the cholesterol-lowering drugs known as the statins may also decrease prostate cancer risk. Sterilization by vasectomy may increase the risk of prostate cancer, though there are conflicting data. Men who are circumcised may have decreased rates of prostate cancer. More frequent ejaculation also may decrease a man's risk of prostate cancer. One study showed that men who ejaculated five times a week in their 20s had a decreased rate of prostate cancer. Infection or inflammation of the prostate (prostatitis) may increase the chance for prostate cancer. In particular, infection with the sexually transmitted infections chlamydia, gonorrhea, and syphilis seem to increase risk. Finally, obesity and elevated blood levels of testosterone may increase the risk for prostate cancer.
Screening
Main article: Prostate cancer screeningProstate cancer screening is an attempt to find unsuspected cancers. Screening tests may lead to more specific follow-up tests such as a biopsy, where small pieces of the prostate are removed for closer study. As of 2005 prostate cancer screening options include the digital rectal exam and the prostate specific antigen (PSA) blood test. Screening for prostate cancer is controversial because it is not clear if the benefits of screening outweigh the risks of follow-up diagnostic tests and cancer treatments.
Prostate cancer is a slow-growing cancer, very common among older men. In fact, most prostate cancers never grow to the point where they cause symptoms, and most men with prostate cancer die of other causes before prostate cancer impacts their lives. The PSA screening test may detect these small cancers that would never become life threatening. Doing the PSA test in these men may lead to overdiagnosis, including additional testing and treatment. Follow-up tests, such as prostate biopsy, may cause pain, bleeding and infection. Prostate cancer treatments may cause urinary incontinence and erectile dysfunction. Therefore, it is essential that the risks and benefits of diagnostic procedures and treatment be carefully considered before PSA screening.
Prostate cancer screening generally begins after age fifty, but may be offered earlier in black men or men with a strong family history of prostate cancer. Although there is no officially recommended cutoff, many health care providers stop monitoring PSA in men who are older than 75 years old because of concern that prostate cancer therapy may do more harm than good as age progresses and life expectancy decreases.
Digital rectal examination
Digital rectal examination (DRE) is a procedure where the examiner inserts a gloved, lubricated finger into the rectum to check the size, shape, and texture of the prostate. Areas which are irregular, hard or lumpy need further evaluation, since they may contain cancer. The DRE only evaluates the back of the prostate, but fortunately, 85% of prostate cancers arise in this part of the prostate. Prostate cancer which can be felt on DRE is generally more advanced. The use of DRE has never been shown to prevent prostate cancer deaths when used as the only screening test.
Prostate specific antigen
The PSA test measures the blood level of prostate-specific antigen, a chemical produced by the prostate. PSA levels under 4 ng/mL (nanograms per milliliter) are generally considered normal, while levels over 10 ng/mL are considered abnormal. PSA levels between 4 and 10 ng/mL are considered borderline. However, PSA is not a perfect test. Some men with prostate cancer do not have an elevated PSA, while some men with an elevated PSA do not have prostate cancer.
PSA levels can change for many reasons other than cancer. Two common causes of high PSA levels are enlargement of the prostate (benign prostatic hypertrophy (BPH)) and infection in the prostate (prostatitis). PSA levels are lowered in men who use medications used to treat BPH or baldness. These medications, finasteride (marketed as Proscar or Propecia) and dutasteride (marketed as Avodart), may decrease the PSA levels by 50 percent or more.
Several other ways of evaluating the PSA have been developed to avoid the shortcomings of simple PSA screening. The rate of rise of the PSA over time, called the PSA velocity, has been used to evaluate men with borderline PSA levels (between 4 and 10 ng/ml) but, as of 2005, has not proven to be an effective screening test. Comparing the PSA level with the size of the prostate, as measured by ultrasound or magnetic resonance imaging, has also been studied. This comparison, called PSA density, is both costly and, as of 2005, has not proven to be an effective screening test. PSA in the blood may either be free or bound to other proteins. Measuring the amount of PSA which is free or bound may provide additional screening information, but as of 2005, questions regarding the usefulness of these measurements limit their widespread use.
Confirming the diagnosis

When a man has symptoms of prostate cancer, or a screening test indicates an increased risk for cancer, more invasive evaluation is offered. The only test which can fully confirm the diagnosis of prostate cancer is a biopsy, the removal of small pieces of the prostate for microscopic examination. However, prior to a biopsy, several other tools may be used to gather more information about the prostate and the urinary tract. Cystoscopy shows the urinary tract from inside the bladder, using a thin, flexible camera tube inserted down the penis. Transrectal ultrasonography creates a picture of the prostate using sound waves from a probe in the rectum.
If cancer is suspected, a biopsy is offered. During a biopsy a urologist obtains tissue samples from the prostate via the rectum. A biopsy gun inserts and removes special hollow-core needles (usually three to six on each side of the prostate) in less than a second. The tissue samples are then examined under a microscope to determine whether cancer cells are present, and to evaluate the microscopic features (or Gleason score) of any cancer found. Prostate biopsies are routinely done on an outpatient basis and rarely require hospitalization. Fifty-five percent of men report discomfort during prostate biopsy.
Staging
Main article: Prostate cancer stagingAn important part of evaluating prostate cancer is determining the stage, or how far the cancer has spread. Knowing the stage helps define prognosis and is useful when selecting therapies. The most common system is the four-stage TNM system (abbreviated from Tumor/Nodes/Metastases). Its components include the size of the tumor, the number of involved lymph nodes, and the presence of any other metastases. The microscopic appearance of the prostate, quantified as the Gleason score, is also incorporated into the staging system. The Whitmore-Jewett stage is another method sometimes used.
The most important distinction made by any staging system is whether or not the cancer is still confined to the prostate. In the TNM system, clinical T1 and T2 cancers are found only in the prostate, while T3 and T4 cancers have spread elsewhere. Several tests can be used to look for evidence of spread. These include computed tomography to evaluate spread within the pelvis, bone scans to look for spread to the bones, and endorectal coil magnetic resonance imaging to closely evaluate the prostatic capsule and the seminal vesicles.
After a prostate biopsy, a pathologist looks at the samples under a microscope. If cancer is present, the pathologist reports the grade of the tumor. The grade tells how much the tumor tissue differs from normal prostate tissue and suggests how fast the tumor is likely to grow. The Gleason system is used to grade prostate tumors from 2 to 10, where a Gleason score of 10 indicates the most abnormalities. The pathologist assigns a number from 1 to 5 for the most common pattern observed under the microscope, then does the same for the second most common pattern. The sum of these two numbers is the Gleason score. Proper grading of the tumor is critical, since the grade of the tumor is one of the major factors used to determine the treatment recommendation.
Treatment
Treatment for prostate cancer may involve watchful waiting, surgery, radiation therapy, chemotherapy, cryosurgery, hormonal therapy, or some combination. Which option is best depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors are the man's age, his general health, and his feelings about potential treatments and their possible side effects. Because all treatments can have significant side effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations.
If the cancer has spread beyond the prostate, treatment options significantly change, so most doctors who treat prostate cancer use a variety of nomograms to predict the probability of spread. Treatment by watchful waiting, radiation therapy, cryosurgery, and surgery are generally offered to men whose cancer remains within the prostate. Hormonal therapy and chemotherapy are often reserved for disease which has spread beyond the prostate. However, there are exceptions: radiation therapy may be used for some advanced tumors, and hormonal therapy is used for some early stage tumors. Cryotherapy, hormonal therapy, and chemotherapy may also be offered if initial treatment fails and the cancer progresses.
Watchful waiting
Watchful waiting, also called "active surveillance," refers to observation and regular monitoring without invasive treatment. Watchful waiting is often used when an early stage, slow-growing prostate cancer is found in an older man. Watchful waiting may also be suggested when the risks of surgery, radiation therapy, or hormonal therapy outweigh the possible benefits. Other treatments can be started if symptoms develop, or if there are signs that the cancer growth is accelerating. Most men who choose watchful waiting for early stage tumors eventually have signs of tumor progression, and they may need to begin treatment within three years. Although men who choose watchful waiting avoid the risks of surgery and radiation, they may also reduce their chances to prevent spread of the cancer. Additional health problems that develop with advancing age during the observation period can also make it harder to undergo surgery and radiation therapy.
Surgery
Surgical removal of the prostate, or prostatectomy, is a common treatment either for early stage prostate cancer, or for cancer which has failed to respond to radiation therapy. The most common type is radical retropubic prostatectomy, when the surgeon removes the prostate through an abdominal incision. Another type is radical perineal prostatectomy, when the surgeon removes the prostate through an incision in the perineum, the skin between the scrotum and anus. Prostatectomy can cure about seventy percent of cases of prostate cancer.
Radical prostatectomy is highly effective for tumors which have not spread beyond the prostate. However, it may cause nerve damage that significantly alters the quality of life of the prostate cancer survivor. The most common serious complications are loss of urinary control and impotence. As many as forty percent of men will be left with some urinary incontinence, usually in the form of leakage when they sneeze, cough or laugh. Impotence is also a common problem. Although penile sensation and the ability to achieve orgasm usually remain intact, erection and ejaculation are often impaired. Medications such as sildenafil (Viagra), tadalafil (Cialis), or vardenafil (Levitra) may restore some degree of potency. In some men with smaller cancers, a more limited "nerve-sparing" technique may help avoid urinary incontinence and impotence.
Radical prostatectomy has traditionally been used alone when the cancer is small. However, courses of hormone therapy prior to surgery may increase cure rates and are currently being studied. Surgery may also be offered when a cancer is not responding to radiation therapy. However, because radiation therapy causes tissue changes, prostatectomy after radiation has a higher risk of complications.
Transurethral resection of the prostate, commonly called a "TURP," is a surgical procedure performed when the tube from the bladder to the penis (urethra) is blocked by prostate enlargement. A small tube (cystoscope) is placed into the penis and the blocking prostate is cut away.
In metastatic disease, where cancer has spread beyond the prostate, removal of the testicles (called orchiectomy) may be done to decrease testosterone levels and control cancer growth. (See hormonal therapy, below).
Radiation therapy

Radiation therapy, also known as radiotherapy, uses X-rays to kill prostate cancer cells. X-rays are a type of ionizing radiation that can damage or destroy the DNA crucial to cancer cell growth. Two different kinds of radiation therapy are used in prostate cancer treatment: external beam radiation therapy and brachytherapy.
External beam radiation therapy uses a linear accelerator to produce high-energy X-rays which are directed in a beam towards the prostate. Several different angles are used so that the skin and other tissues around the prostate receive less radiation exposure than the prostate itself. External beam radiation therapy is generally given over several weeks, with daily visits to a radiation therapy center.

Brachytherapy involves the placement of about 100 small "seeds" containing radioactive material (such as iodine-125 or palladium-103) with a needle through the skin of the perineum directly into the tumor. These seeds emit lower-energy X-rays which are only able to travel a short distance. Brachytherapy seeds will stay in the prostate permanently, but men with implanted seeds are not at risk of exposing others to radiation.
Radiation therapy for prostate cancer is commonly used in two different situations. It may be used instead of surgery for early cancers, and it may also be used in advanced stages of prostate cancer to treat painful bone metastases. Other less common applications are after surgery which did not fully remove the cancer and when cancer is compressing the spinal cord.
Radiation therapy is often offered to men with medical problems which make surgery more risky. Radiation therapy appears to cure small tumors that are confined to the prostate just about as well as surgery. However, as of 2005 some issues remain unresolved, such as whether radiation should be given to the rest of the pelvis, how much the absorbed dose should be, and whether hormonal therapy should be given at the same time.
Complications of radiation therapy are common in the weeks immediately after therapy. Both types of radiation therapy may cause diarrhea and rectal bleeding due to radiation proctitis, as well as urinary incontinence and impotence. Symptoms tend to improve over time. Men who have undergone external beam radiation therapy will have a higher risk of later developing colon cancer and bladder cancer.
Cryosurgery
Cryosurgery is another method of treating prostate cancer. It is less invasive than radical prostatectomy, and general anesthesia is less commonly used. Under ultrasound guidance, metal rods are inserted through the skin of the perineum into the prostate. Liquid nitrogen is used to cool the rods, freezing the surrounding tissue at −196 °C (−320 °F). As the water within the prostate cells freezes, the cells die. The urethra is protected from freezing by a catheter filled with warm liquid. Cryosurgery generally causes fewer problems with urinary control than other treatments, but impotence occurs up to ninety percent of the time. When used as the initial treatment for prostate cancer, cryosurgery is not as effective as surgery or radiation. However, cryosurgery is potentially better than radical prostatectomy for recurrent cancer following radiation therapy.
Hormonal therapy

Hormonal therapy uses medications or surgery to block prostate cancer cells from getting dihydrotestosterone (DHT), a hormone produced in the prostate and required for the growth and spread of most prostate cancer cells. Blocking DHT often causes prostate cancer to stop growing and even shrink. However, hormonal therapy rarely cures prostate cancer because cancers which initially respond to hormonal therapy typically become resistant after one to two years. Hormonal therapy is therefore usually used when cancer has spread from the prostate. It may also be given to certain men undergoing radiation therapy or surgery to help prevent return of their cancer.
Hormonal therapy for prostate cancer targets the pathways the body uses to produce DHT. A feedback loop involving the testicles, the hypothalamus, and the pituitary, adrenal, and prostate glands controls the blood levels of DHT. First, low blood levels of DHT stimulate the hypothalamus to produce gonadotropin releasing hormone (GnRH). GnRH then stimulates the pituitary gland to produce luteinizing hormone (LH), and LH stimulates the testicles to produce testosterone. Finally, testosterone from the testicles and dehydroepiandrosterone from the adrenal glands stimulate the prostate to produce more DHT. Hormonal therapy can decrease levels of DHT by interrupting this pathway at any point.
There are several forms of hormonal therapy:
- Orchiectomy is surgery to remove the testicles. Because the testicles make most of the body's testosterone, after orchiectomy testosterone levels drop. Now the prostate not only lacks the testosterone stimulus to produce DHT, but also it does not have enough testosterone to transform into DHT.
- Antiandrogens are medications such as flutamide, bicalutamide, nilutamide, and cyproterone acetate which directly block the actions of testosterone and DHT within prostate cancer cells.
- Medications which block the production of adrenal androgens such as DHEA include ketoconazole and aminoglutethimide. Because the adrenal glands only make about 5 percent of the body's androgens, these medications are generally used only in combination with other methods that can block the 95 percent of androgens made by the testicles. These combined methods are called total androgen blockade (TAB). TAB can also be achieved using antiandrogens.
- GnRH action can be interrupted in one of two ways. GnRH antagonists suppress the production of GnRH directly, while GnRH agonists suppress GnRH indirectly. GnRH antagonists include the estrogens, diethylstilbestrol and estradiol, and the medication abarelix. GnRH agonists include leuprolide, goserelin, triptorelin, and buserelin. Initially, these medications increase the production of LH. However, because the constant supply of the medication does not match the body's natural production rhythm, production of both LH and GnRH decreases after a few weeks.
As of 2005 the most successful hormonal treatments are orchiectomy and GnRH agonists. Despite their higher cost, GnRH agonists are often chosen over orchiectomy for cosmetic and emotional reasons. Eventually, total androgen blockade may prove to be better than orchiectomy or GnRH agonists used alone.
Each treatment has disadvantages which limit its use in certain circumstances. Although orchiectomy is a low-risk surgery, the psychological impact of removing the testicles can be significant. The loss of testosterone also causes hot flashes, weight gain, loss of libido, enlargement of the breasts (gynecomastia), impotence and osteoporosis. GnRH agonists eventually cause the same side effects as orchiectomy but may cause worse symptoms at the beginning of treatment. When GnRH agonists are first used, testosterone surges can lead to increased bone pain from metastatic cancer, so antiandrogens or abarelix are often added to blunt these side effects. Estrogens are not commonly used because they increase the risk for cardiovascular disease and blood clots. The antiandrogens do not generally cause impotence and usually cause less loss of bone and muscle mass. Ketoconazole can cause liver damage with prolonged use, and aminoglutethimide can cause skin rashes.
Palliative care
Palliative care for advanced stage prostate cancer focuses on extending life and relieving the symptoms of metastatic disease. Chemotherapy may be offered to slow disease progression and postpone symptoms. The most commonly used regimen combines the chemotherapeutic drug docetaxel with a corticosteroid such as prednisone. Bisphosphonates such as zoledronic acid have been shown to delay skeletal complications such as fractures or the need for radiatoin therapy in patients with hormone-refractory metastatic prostate cancer.
Bone pain due to metastatic disease is treated with opioid pain relievers such as morphine and oxycodone. External beam radiation therapy directed at bone metastases may provide pain relief. Injections of certain radioisotopes, such as strontium-89, phosphorus-32, or samarium-153, also target bone metastases and may help relieve pain.
Prognosis
Prostate cancer rates are higher and prognosis poorer in Western societies than the rest of the world. Many of the risk factors for prostate cancer are more prevalent in the Western world, including longer life expectancy, diets high in animal fats, and more access to screening programs. Prostate cancer is the ninth most common cancer in the world, but is the number one non-skin cancer in United States men. Prostate cancer affected eighteen percent of American men and caused death in three percent in 2005. In Japan, death from prostate cancer was one-fifth to one-half the rates as the United States and Europe in the 1990s. In India in the 1990s, half of the people with prostate cancer confined to the prostate cancer died by ten years. African-American men have 50-60 times more prostate cancer and prostate cancer deaths than men in Shanghai, China In Nigeria, two percent of men develop prostate cancer and 64% are dead after two years.
In patients who undergo treatment, the most important clinical prognostic indicators of disease outcome are stage, pre-therapy PSA level and Gleason score. In general, the higher the grade and the stage, the poorer the prognosis. Nomograms can be used to calculate the estimated risk of the individual patient. The predictions are based on the experience of large groups of patients suffering from cancers at various stages.
Prevention
Prostate cancer risk can be improved by modifying known risk factors for prostate cancer, such as decreasing intake of animal fat. Several medications and vitamins may also help prevent prostate cancer. Two dietary supplements, vitamin E and selenium, may help prevent prostate cancer when taken daily. Estrogens from soybeans and other plant sources (called phytoestrogens) may also help prevent prostate cancer. The selective estrogen receptor modulator drug toremifene has shown promise in early trials. Two medications which block the conversion of testosterone to dihydrotestosterone, finasteride and dutasteride, have also shown some promise. As of 2005 the use of these medications for primary prevention is still in the testing phase, and they are not widely used for this purpose.
History
Although the prostate was first described by Venetian anatomist Niccolò Massa in 1536, and illustrated by Flemish anatomist Andreas Vesalius in 1538, prostate cancer was not identified until 1853. Prostate cancer was initially considered a rare disease, probably because of shorter life expectancies and poorer detection methods in the 19th century. The first treatments of prostate cancer were surgeries to relieve urinary obstruction. Removal of the entire gland (radical perineal prostatectomy) was first performed in 1904 by Hugh Young at Johns Hopkins Hospital. Surgical removal of the testes (orchiectomy) to treat prostate cancer was first performed in the 1890s, but with limited success. Transurethral resection of the prostate (TURP) replaced radical prostatectomy for symptomatic relief of obstruction in the middle of the 20th century because it could better preserve penile erectile function. Radical retropubic prostatectomy was developed in 1983 by Patrick Walsh.

This surgical approach allowed for removal of the prostate and lymph nodes with maintenance of penile function.
In 1941 Charles B. Huggins published studies in which he used estrogen to oppose testosterone production in men with metastatic prostate cancer. This discovery of "chemical castration" won Huggins the 1966 Nobel Prize in Physiology or Medicine. The role of the hormone GnRH in reproduction was determined in 1971 by Andrzej W. Schally, and receptor agonists such as leuprolide and goserelin were quickly developed to use this knowledge to treat prostate cancer. In 1977 Schally also won the Nobel Prize in Physiology or Medicine for his work.
Radiation therapy for prostate cancer was first developed in the early 20th century and initially consisted of intraprostatic radium implants. External beam radiation became more popular as stronger radiation sources became available in the middle of the 20th century. Brachytherapy with implanted seeds was first described in 1983. Systemic chemotherapy for prostate cancer was first studied in the 1970s. The initial regimen of cyclophosphamide and 5-fluorouracil was quickly joined by multiple regimens using a host of other systemic chemotherapy drugs.
References
- American Cancer Society webpage. Detailed Guide: prostate cancer. Found at http://www.cancer.org/docroot/CRI/CRI_2_3x.asp?rnav=cridg&dt=36
- Aumüller, G. Prostate Gland and Seminal Vesicles. Springer-Verlag Berlin-Heidelberg. 1979.
- Moore, K., Dalley, A. Clinically Oriented Anatomy. Lippincott Williams & Wilkins, Baltimore, Maryland. 1999.
- Steive, H. Männliche Genitalorgane. In: Handbuch der mikroskopischen Anatomie des Menschen. Vol. VII Part 2, pp. 1-399. Berlin: Springer 1930.
- Miller, DC, Hafez, KS, Stewart, A, et al. Prostate carcinoma presentation, diagnosis, and staging: an update form the National Cancer Data Base. Cancer 2003; 98:1169. PMID 12973840
- van der Cruijsen-Koeter IW, Vis AN, Roobol MJ, Wildhagen MF, de Koning HJ, van der Kwast TH, Schroder FH. Comparison of screen detected and clinically diagnosed prostate cancer in the European randomized study of screening for prostate cancer, section rotterdam. Urol. 2005 Jul;174(1):121-5. PMID 15947595
- Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Kaplan RS. Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999 Jun 16;91(12):1017-24. PMID 10379964
- Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, Tulinius H. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977 Nov 15;20(5):680-8. PMID 924691
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005 Jan-Feb;55(1):10-30. Erratum in: CA Cancer J Clin. 2005 Jul-Aug;55(4):259. PMID 15661684
- Hoffman RM; Gilliland FD; Eley JW; Harlan LC; Stephenson RA; Stanford JL; Albertson PC; Hamilton AS; Hunt WC; Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001 Mar 7;93(5):388-95. PMID 11238701
- Steinberg GD; Carter BS; Beaty TH; Childs B; Walsh PC. Family history and the risk of prostate cancer. Prostate. 1990;17(4):337-47. PMID 2251225
- Lichtenstein P; Holm NV; Verkasalo PK; Iliadou A; Kaprio J; Koskenvuo M; Pukkala E; Skytthe A; Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000 Jul 13;343(2):78-85. PMID 10891514
- Struewing JP; Hartge P; Wacholder S; Baker SM; Berlin M; McAdams M; Timmerman MM; Brody LC; Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997 May 15;336(20):1401-8. PMID 9145676
- Schulman CC; Ekane S; Zlotta AR. Nutrition and prostate cancer: evidence or suspicion? Urology. 2001 Sep;58(3):318-34. PMID 11549473
- Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005 Jul 6;97(13):975-80. PMID 15998950
- Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005 Aug 15;162(4):318-25. Epub 2005 Jul 13. PMID 16014776
- Giovannucci E, Tosteson TD, Speizer FE, Ascherio A, Vessey MP, Colditz GA. A retrospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993 Feb 17;269(7):878-82. PMID 8123059
- Template:Journal reference issue PMID 3471995
- Giles GG; Severi G; English DR; McCredie MR; Borland R; Boyle P; Hopper JL. Sexual factors and prostate cancer. BJU Int. 2003 Aug;92(3):211-6. PMID 12887469
- Dennis LK; Lynch CF; Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002 Jul;60(1):78-83. PMID 12100928
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625-38. PMID 12711737
- Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996 Aug 21;88(16):1118-26. PMID 8757191
- Grubb RL, Roehl KA, Antenor JA, Catalona WJ. Results of compliance with prostate cancer screening guidelines. J Urol. 2005 Aug;174(2):668-72; discussion 672. PMID 16006944
- Chodak GW, Keller P, Schoenberg HW. Assessment of screening for prostate cancer using the digital rectal examination. J Urol. 1989 May;141(5):1136-8. PMID 2709500
- Krahn MD, Mahoney JE, Eckman MH, Trachtenberg J, Pauker SG, Detsky AS. Screening for prostate cancer. A decision analytic view. JAMA. 1994 Sep 14;272(10):773-80. PMID 7521400
- Roobol MJ, Kranse R, de Koning HJ, Schroder FH. Prostate-specific antigen velocity at low prostate-specific antigen levels as screening tool for prostate cancer: results of second screening round of ERSPC (ROTTERDAM). Urology. 2004 Feb;63(2):309-13; discussion 313-5. PMID 14972478
- Catalona WJ, Richie JP, deKernion JB, Ahmann FR, Ratliff TL, Dalkin BL, Kavoussi LR, MacFarlane MT, Southwick PC. Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994 Dec;152(6 Pt 1):2031-6. PMID 7525994
- Hoffman RM, Clanon DL, Littenberg B, Frank JJ, Peirce JC. Using the free-to-total prostate-specific antigen ratio to detect prostate cancer in men with nonspecific elevations of prostate-specific antigen levels. J Gen Intern Med. 2000 Oct;15(10):739-48. PMID 11089718
- Partin AW; Brawer MK; Bartsch G; Horninger W; Taneja SS; Lepor H; Babaian R; Childs SJ; Stamey T; Fritsche HA; Sokoll L; Chan DW; Thiel RP; Cheli CD. Complexed prostate specific antigen improves specificity for prostate cancer detection: results of a prospective multicenter clinical trial.J Urol. 2003 Nov;170(5):1787-91. PMID 14532777
- Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schroder FH. Short-term effects of population-based screening for prostate cancer on health-related quality of life.J Natl Cancer Inst. 1998 Jun 17;90(12):925-31. PMID 9637143
- Wu H, Sun L, Moul JW, Wu HY, McLeod DG, Amling C, Lance R, Kusuda L, Donahue T, Foley J, Chung A, Sexton W, Soderdahl D. Watchful waiting and factors predictive of secondary treatment of localized prostate cancer.J Urol. 2004 Mar;171(3):1111-6. PMID 14767282
- Gerber GS, Thisted RA, Scardino PT, Frohmuller HG, Schroeder FH, Paulson DF, Middleton AW Jr, Rukstalis DB, Smith JA Jr, Schellhammer PF, Ohori M, Chodak GW. Results of radical prostatectomy in men with clinically localized prostate cancer. JAMA. 1996 Aug 28;276(8):615-9. PMID 8773633
- Template:Journal reference issue PMID 12756082
- Perez CA, Hanks GE, Leibel SA, Zietman AL, Fuks Z, Lee WR. Localized carcinoma of the prostate (stages T1B, T1C, T2, and T3). Review of management with external beam radiation therapy. Cancer. 1993 Dec 1;72(11):3156-73. Review. PMID 7694785
- Lawton CA, Won M, Pilepich MV, Asbell SO, Shipley WU, Hanks GE, Cox JD, Perez CA, Sause WT, Doggett SR, et al. Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys. 1991 Sep;21(4):935-9. PMID 1917622
- Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000 Jan 15;88(2):398-406. PMID 10640974
- Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002 Aug;60(2 Suppl 1):3-11. PMID 12206842
- Robson M, Dawson N.How is androgen-dependent metastatic prostate cancer best treated? Hematol Oncol Clin North Am. 1996 Jun;10(3):727-47. Review. PMID 8773508
- Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben-Josef E, Middleton R, Porterfield H, Sharp SA, Smith TJ, Taplin ME, Vogelzang NJ, Wade JL Jr, Bennett CL, Scher HI; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004 Jul 15;22(14):2927-41. Erratum in: J Clin Oncol. 2004 Nov 1;22(21):4435. PMID 15184404
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1502-12. PMID 1547021
- Template:Journal reference issue PMID 12359855
- Jemal A; Murray T; Ward E; Samuels A; Tiwari RC; Ghafoor A; Feuer EJ; Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005 Jan-Feb;55(1):10-30. Erratum in: CA Cancer J Clin. 2005 Jul-Aug;55(4):259. PMID 15661684
- Wakai K. Nippon Rinsho. 2005 Feb;63(2):207-12. Review. Japanese. PMID 15714967
- Yeole BB, Sunny L. Population based survival from prostate cancer in Mumbai (Bombay), India. Indian J Cancer. 2001 Jun-Dec;38(2-4):126-32. PMID 1259345
- Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000 Jan 1;85(1):60-7. PMID 10585584
- Osegbe DN. Prostate cancer in Nigerians: facts and nonfacts. J Urol. 1997 Apr;157(4):1340-3. PMID 9120935
- Template:Journal reference issue PMID 14571407
- Template:Journal reference issue PMID 12778049
- Steiner, MS, Pound, CR, Gingrich, JR, et al. Acapodene (GTx-006) reduces high-grade prostatic intraepithelial neoplasia in phase II clinical trial (abstract). Proc Am Soc Clin Oncol 2002; 21:180a.
- Price, D, Stein, B, Goluboff, E, et al. Double-blind, placebo-controlled trial of toremifene for the provention of prostate cancer in men with high-grade prostatic intrapeithelial neoplasia (abstract). J Clin Oncol 2005; 23:106s.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA Jr. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003 Jul 17;349(3):215-24. PMID 12824459
- Andriole GL, Roehrborn C, Schulman C, Slawin KM, Somerville M, Rittmaster RS. Effect of dutasteride on the detection of prostate cancer in men with benign prostatic hyperplasia. Urology. 2004 Sep;64(3):537-41; discussion 542-3. PMID 15351586
- Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, Hursting SD. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer. 1999;33(1):20-5. Erratum in: Nutr Cancer 2000;36(2):243. PMID 10227039
- Adams, J. The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. Lancet 1, 393 (1853).
- Lytton, B. Prostate cancer: a brief history and the discovery of hormonal ablation treatment. J. Urol. 165, 1859-1862
- Young, H. H. Four cases of radical prostatectomy. Johns Hopkins Bull. 16, 315 (1905).
- Walsh, P. C., Lepor, H. & Eggleston, J. C. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate 4, 473-485 (1983). PMID 6889192
- Huggins, C. B. & Hodges, C. V. Studies on prostate cancer: 1. The effects of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 203 (1941).
- Schally, A. V., Kastin, A. J. & Arimura, A. Hypothalamic FSH and LH-regulating hormone. Structure, physiology and clinical studies. Fertil. Steril. 22, 703-721 (1971).
- Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, Comaru-Schally AM, Schally AV. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1658-62 PMID 6461861
- Denmeade SR, Isaacs JT. A History of Prostate Cancer Treatment. Nature Reviews Cancer 2, 389-396 (2002). PMID 12044015
- Scott, W. W. et al. Chemotherapy of advanced prostatic carcinoma with cyclophosphamide or 5-fluorouracil: results of first national randomized study. J. Urol. 114, 909-911 (1975). PMID 1104900
External links
- Prostate Cancer Foundation
- Information about prostate cancer on prostate.com
- IGF1R gene may increase prostate cancer treatment
- National Institute on Aging Information Center
- National Kidney and Urologic Diseases Information Clearinghouse
- Prostate Cancer Survivor One survivor's story
- Many NCI publications may be viewed or ordered on the Internet at http://cancer.gov/publications
Patient support sources:
- news://alt.support.cancer.prostate is a newsgroup quite active with men currently dealing with the disease.
- nonprofit prostate cancer website updated weekly
- largest patient supported non-commercial website for all aspects of prostate cancer