This is an old revision of this page, as edited by WolfmanSF (talk | contribs) at 23:25, 30 May 2010 (→Natural reservoirs: punctuation). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:25, 30 May 2010 by WolfmanSF (talk | contribs) (→Natural reservoirs: punctuation)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) For other uses, see Ebola (disambiguation). Medical condition| Ebola | |
|---|---|
| Specialty | Infectious diseases |
| Ebola | |
|---|---|
| |
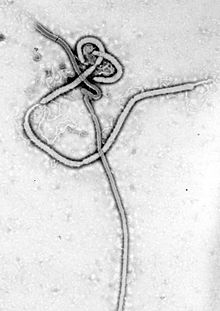
| Ebola virus electron micrograph | |
| Virus classification | |
| Group: | Group V ((−)ssRNA) |
| Order: | Mononegavirales |
| Family: | Filoviridae |
| Genus: | Ebolavirus |
| Species | |
|
Ivory Coast ebolavirus | |
Ebola is the virus Ebolavirus (EBOV), a viral genus, and the disease Ebola hemorrhagic fever (EHF), a viral hemorrhagic fever (VHF). The virus is named after the Ebola River Valley in the Democratic Republic of the Congo (formerly Zaire), which is near the site of the first recognized outbreak in 1976 at a mission hospital run by Flemish nuns. It remained largely obscure until 1989 when several widely publicized outbreaks occurred among monkeys in the United States.
The virus interferes with the endothelial cells lining the interior surface of blood vessels and with coagulation. As the blood vessel walls become damaged and the platelets are unable to coagulate, patients succumb to hypovolemic shock. Ebola is transmitted through bodily fluids, while conjunctiva exposure may also lead to transmission.
There are five recognized species within the ebolavirus genus, which have a number of specific strains. The Zaire virus is the type species, which is also the first discovered and the most lethal. Electron micrographs show long filaments, characteristic of the Filoviridae viral family.
Classification
The genera Ebolavirus and Marburgvirus were originally classified as the species of the now-obsolete Filovirus genus. In March 1998, the Vertebrate Virus Subcommittee proposed in the International Committee on Taxonomy of Viruses (ICTV) to change the Filovirus genus to the Filoviridae family with two specific genera: Ebola-like viruses and Marburg-like viruses. This proposal was implemented in Washington, D.C. as of April, 2001 and in Paris as of July 2002. In 2000, another proposal was made in Washington, D.C. to change the "-like viruses" to "-virus" resulting in today's Ebolavirus and Marburgvirus.

Rates of genetic change are one hundred times slower than Influenza A in humans, but on the same magnitude of that of Hepatitis B. Using these rates, the Ebolavirus and Marburgvirus are estimated to have diverged several thousand years ago.
- Zaire virus (ZEBOV)
- The Zaire virus, formerly named Zaire Ebola Virus, has the highest case-fatality rate, up to 90% in some epidemics, with an average case fatality rate of approximately 83% over 27 years. There have been more outbreaks of Zaire ebolavirus than any other species. The first outbreak took place on 26 August 1976 in Yambuku. Mabalo Lokela, a 44-year-old schoolteacher, became the first recorded case. The symptoms resembled malaria, and subsequent patients received quinine. The initial transmission was believed to be due to reuse of the needle for Lokela's injection without sterilization. Subsequent transmission was also due to lack of barrier nursing and the traditional burial preparation method, which involves washing and gastrointestinal tract cleansing.
- Sudan ebolavirus (SEBOV)
- The virus was the second species of Ebola emerging simultaneous with the Zaire virus. It was believed to have originated amongst cotton factory workers in Nzara, Sudan, with the first case reported as a worker exposed to a potential natural reservoir. Scientists tested all animals and insects in response to this; however, none tested positive for the virus. The carrier is still unknown. The lack of barrier nursing facilitated the spread of the disease. The most recent outbreak occurred in May 2004. 20 confirmed cases were reported in Yambio County, Sudan, with five deaths resulting. The average fatality rates for were 54% in 1976, 68% in 1979, and 53% in 2000 and 2001.
- Reston ebolavirus (REBOV)
- Discovered during an outbreak of Simian hemorrhagic fever virus (SHFV) in crab-eating macaques from Hazleton Laboratories (now Covance) in 1989. Since the initial outbreak in Reston, Virginia, it has emerged in Siena Italy, Texas, and among pigs in the Philippines. Despite its status as a Level-4 organism, it is non-pathogenic to humans however hazardous in monkeys.
- Cote d'Ivoire ebolavirus (CIEBOV)
- Also referred to as Ivory Coast ebolavirus and Tai ebolavirus, it was first discovered among chimpanzees from the Tai Forest in Côte d'Ivoire, Africa on 1 November 1994. Necropsies showed blood within the heart to be brown, no obvious marks were seen on the organs, and one necropsy displayed lungs filled with blood. Studies of tissues taken from the chimpanzees showed results similar to human cases during the 1976 Ebola outbreaks in Zaire and Sudan. As more dead chimpanzees were discovered, many tested positive for Ebola using molecular techniques. The source of contamination was believed to be the meat of infected Western Red Colobus monkeys, upon which the chimpanzees preyed. One of the scientists performing the necropsies on the infected chimpanzees contracted Ebola. She developed symptoms similar to those of dengue fever approximately a week after the necropsy, and was transported to Switzerland for treatment. She was discharged from hospital after two weeks and had fully recovered six weeks after the infection.
- Bundibugyo ebolavirus
- On November 24, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the CDC, the World Health Organization confirmed the presence of the new species. On 20 February 2008, the Uganda Ministry officially announced the end of the epidemic in Bundibugyo with the last infected person discharged on 8 January 2008. Ugandan officials confirmed a total of 149 cases of this new Ebola species, with 37 deaths attributed to the strain (24.83%).
Signs and symptoms
The incubation period ranges from 2–21 days, although it is generally 5–18 days,. Illness is characterized by the rapid onset of fever, malaise, muscle pain, headache, and the inflammation of the pharynx. Six days following vomiting and bloody diarrhea, individuals may develop maculopapular rash with bleeding at needle sites and bodily orifices.
Reston ebolavirus is non-pathogenic to humans and individuals often do not show any symptoms, although it is fatal in monkeys. There is only one known case of Ivory Coast ebolavirus. There has been only one outbreak of Bundibugyo ebolavirus. Zaire virus, then Sudan ebolavirus, are the most common. Symptoms include: abdominal pain (60-80%), fever (90%-100%), headache (40%-90%), bloody vomit (10%-40%), Maculopapular rash (5%-20%), malaise (75%-85%), joint and muscle pain (40%-80%), inflammation of the pharynx (20%-40%), blood fails to clot (71%-78%), chest pain (SEBOV only 83%), CNS involvement (rare), dry and sore throat (63%), hemorrhagic diathesis (71%-78%), hiccups (15%), non-bloody diarrhea (81%), vomiting (59%). Purpura, petechia, sclerotic arterioles, and low blood-pressure are characteristic as the disease progresses.
Virology
Structure
Electron micrographs of members of genus Ebolavirus show them to have the characteristic thread-like structure of a filovirus. EBOV VP30 is around 288 amino acids long. The virions are tubular in general form but variable in overall shape and may appear as the classic shepherd's crook or eyebolt, as a U or a 6, or coiled, circular, or branched; laboratory techniques, such as centrifugation, may be the origin of some of these formations. Virions are generally 80 nm in diameter with a lipid bilayer anchoring the glycoprotein which projects 7 to 10 nm long spikes from its surface. They are of variable length, typically around 800 nm, but may be up to 1000 nm long. In the center of the virion is a structure called nucleocapsid, which is formed by the helically wound viral genomic RNA complexed with the proteins NP, VP35, VP30, and L. It has a diameter of 80 nm and contains a central channel of 20–30 nm in diameter. Virally encoded glycoprotein (GP) spikes 10 nm long and 10 nm apart are present on the outer viral envelope of the virion, which is derived from the host cell membrane. Between envelope and nucleocapsid, in the so-called matrix space, the viral proteins VP40 and VP24 are located.
Genome
Each virion contains one molecule of linear, single-stranded, negative-sense RNA, 18,959 to 18,961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. It was found that 472 nucleotides from the 3' end and 731 nucleotides from the 5' end are sufficient for replication. It codes for seven structural proteins and one non-structural protein. The gene order is 3′ - leader - NP - VP35 - VP40 - GP/sGP - VP30 - VP24 - L - trailer - 5′; with the leader and trailer being non-transcribed regions, which carry important signals to control transcription, replication, and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins, among them the RNA-dependent RNA polymerase, are necessary to transcribe the viral genome into mRNAs, as well as for replication of the viral genome.
Replication
Being acellular, viruses do not grow through cell division; instead, they use the machinery and metabolism of a host cell to produce multiple copies of themselves, and they assemble in the cell.
- The virus attaches to host receptors through the glycoprotein (GP) surface peplomer and is endocytosed into vesicles in the host cell
- Viral membrane fuses with vesicle membrane, nucleocapsid is released into the cytoplasm
- Encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis (3' - 5') of polyadenylated, monocistronic mRNAs
- Using the host cell's machinery translation of the mRNA into viral proteins occurs
- Viral proteins are processed, glycoprotein precursor (GP0) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. Secreted glycoprotein (sGP) precursor is cleaved to sGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated.
- The newly formed nucleocapsides and envelope proteins associate at the host cell's plasma membrane; budding occurs, destroying the cell.
Pathogenesis
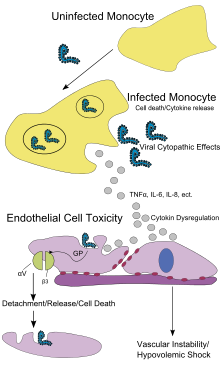
Endothelial cells, mononuclear phagocytes, and hepatocytes are the main targets of infection. After infection, in a secreted glycoprotein (sGP) the Ebola virus glycoprotein (GP) is synthesized. Ebola replication overwhelms protein synthesis of infected cells and host immune defenses. The GP forms a trimeric complex, which binds the virus to the endothelial cells lining the interior surface of blood vessels. The sGP forms a dimeric protein which interferes with the signaling of neutrophils, a type of white blood cell, which allows the virus to evade the immune system by inhibiting early steps of neutrophil activation. The presence of viral particles and cell damage resulting from budding causes the release of cytokines (specifically TNF-α, IL-6, IL-8, etc.), which are the signaling molecules for fever and inflammation. The cytopathic effect, from infection in the endothelial cells, results in a loss of vascular integrity. This loss in vascular integrity is furthered with synthesis of GP, which reduces specific integrins responsible for cell adhesion to the inter-cellular structure, and damage to the liver, which leads to coagulopathy. Without vascular integrity and effective coagulation, blood quickly leaks through the blood vessel leading to hypovolemic shock.
Diagnosis
Before outbreaks are confirmed in areas of weak surveillance on the local or regional levels ebola is often mistaken for malaria, typhoid fever, dysentery, influenza, or various bacterial infections which may be endemic to the region. Learning from the failure response such as the 2000 Uganda outbreak, public health measures such as the WHO's Global Outbreak and Response Network were instituted in areas at high risk. Field laboratories were established in order to confirm cases as to shipping samples to South Africa.
Methods of diagnosis of Ebola include testing saliva and urine samples. Ebola is diagnosed with an Enzyme-Linked ImmunoSorbent Assay (ELISA) test. This diagnosis method has produced potentially ambiguous results during non-outbreak situations. Following Reston, and in an effort to evaluate the original test, Dr. Karl Johnson of the CDC tested San Blas Indians from Central America, who have no history of Ebola infection, and observed a 2% positive result. Other researchers later tested sera from Native Americans in Alaska and found a similar percentage of positive results. To combat the false positives, a more complex test based on the ELISA system was developed by Tom Kzaisek at USAMRIID, which was later improved with Immunofluorescent antibody analysis (IFA). It was however not used during the serosurvey following Reston. These tests are not commercially available. Polymerase Chain Reaction (PCR) technique has been successfully used for detection of Ebola virus PCR Technique for Detection of Ebola Virus by Hänninen 2001
Prevention

In the early stages, Ebola may not be highly contagious. Contact with someone in early stages may not even transmit the disease. As the illness progresses, bodily fluids from diarrhea, vomiting, and bleeding represent a hazard. Due to lack of proper equipment and hygienic practices, large-scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Many areas where the infectious reservoir exists have just these characteristics. In such environments, all that can be done is to immediately cease all needle-sharing or use without adequate sterilization procedures, isolate patients, and observe strict barrier nursing procedures with the use of a medical rated disposable face mask, gloves, goggles, and a gown at all times. This should be strictly enforced for all medical personnel and visitors.
Vaccines have successfully protected non-human primates; however, the six months needed to complete immunization made it impractical in an epidemic. To resolve this, in 2003 a vaccine using an adenoviral (ADV) vector carrying the Ebola spike protein was tested on crab-eating macaques. The monkeys were challenged with the virus twenty-eight days later, and remained resistant. In 2005 a vaccine based on attenuated recombinant vesicular stomatitis virus (VSV) vector carrying either the Ebola glycoprotein or Marburg glycoprotein successfully protected non-human primates, opening clinical trials in humans. By October the study completed the first human trial giving three vaccinations over three months showing capability of safely inducing an immune response. Individuals were followed for a year, and in 2006 a study testing a faster-acting, single shot vaccine began. This study was completed in 2008.
Treatment

There is no standard treatment for Ebola hemorrhagic fever. Treatment is primarily supportive and includes minimizing invasive procedures, balancing electrolytes, and, since patients are frequently dehydrated, replacing lost coagulation factors to help stop bleeding, maintaining oxygen and blood levels, and treating any complicating infections. Convalescent plasma (factors from those that have survived Ebola infection) shows promise as a treatment for the disease. Ribavirin is ineffective. Interferon is also thought to be ineffective. In monkeys, administration of an inhibitor of coagulation (rNAPc2) has shown some benefit, protecting 33% of infected animals from a usually 100% (for monkeys) lethal infection (however, this inoculation does not work on humans). In early 2006, scientists at USAMRIID announced a 75% recovery rate after infecting four rhesus monkeys with Ebolavirus and administering Morpholino antisense drugs. Development of improved Morpholino antisense conjugated with cell penetrating peptides is ongoing.
In May 2010, a group of scientists from the National Emerging Infectious Diseases Laboratories at Boston University announced they had developed a drug that prevented reproduction of the virus in monkeys. The group's leader Thomas Geisbert claimed the results of the group's study showed their experimental treatment resulted in "complete protection" of monkeys from the virus. Virologist Heinz Feldmann hailed the findings of the study as a "milestone" that could be used to combat similar viruses. Geisbert claimed a lack of market interest could impair the development of the treatment for humans, given the comparatively low number of Ebola cases worldwide. The trial on monkeys had been funded by the United States Department of Defense.
Prognosis
Ebola hemorrhagic fever is potentially lethal and encompasses a range of symptoms including fever, vomiting, diarrhea, generalized pain or malaise, and sometimes internal and external bleeding. The span of time from onset of symptoms to death is usually between 2 and 21 days. By the second week of infection, patients will either defervesce (the fever will lessen) or undergo systemic multi-organ failure. Mortality rates are typically high, with the human case-fatality rate ranging from 50–89%, depending on the species or viral strain. The cause of death is usually due to hypovolemic shock or organ failure.
Epidemiology
Natural reservoirs
Between 1976 and 1998, from 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no Ebolavirus was detected apart from some genetic material found in six rodents (Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic. The virus was detected in the carcasses of gorillas, chimpanzees, and duikers during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high mortality from infection in these species makes them unlikely as a natural reservoir.
Plants, arthropods, and birds have also been considered as possible reservoirs; however, bats are considered the most likely candidate. Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed, and they have also been implicated in Marburg infections in 1975 and 1980. Of 24 plant species and 19 vertebrate species experimentally inoculated with Ebolavirus, only bats became infected. The absence of clinical signs in these bats is characteristic of a reservoir species. In a 2002–2003 survey of 1,030 animals which included 679 bats from Gabon and the Republic of the Congo, 13 fruit bats were found to contain Ebolavirus RNA. As of 2005, three fruit bat species (Hypsignathus monstrosus, Epomops franqueti, and Myonycteris torquata) have been identified as carrying the virus while remaining asymptomatic. They are believed to be a natural host species, or reservoir, of the virus.
Reston ebolavirus—unlike its African counterparts—is non-pathogenic in humans. The high mortality among monkeys and its recent emergence in swine, makes them unlikely natural reservoirs.
Transmission
Bats drop partially eaten fruits and pulp, terrestrial mammals such as gorillas and duikers feed on these fallen fruits. This chain of events forms a possible indirect means of transmission from the natural host to animal populations, which have led to research towards viral shedding in the saliva of bats. Fruit production, animal behavior, and other factors vary at different times and places which may trigger outbreaks among animal populations. Transmission between natural reservoirs and humans are rare, and outbreaks are usually traceable to a single index case where an individual has handled the carcass of gorilla, chimpanzee, or duiker. The virus then spreads person-to-person, especially within families, hospitals, and during some mortuary rituals where contact among individuals becomes more likely.
The virus has been confirmed to be transmitted through body fluids. Transmission through oral exposure and through conjunctiva exposure is likely, which have been confirmed in non-human primates. Filoviruses are not naturally transmitted by aerosol. They are, however, highly infectious as breathable 0.8-1.2 micron droplets in laboratory conditions; because of this potential route of infection, these viruses have been classified as Category A biological weapons.
All epidemics of Ebola have occurred in sub-optimal hospital conditions, where practices of basic hygiene and sanitation are often either luxuries or unknown to caretakers and where disposable needles and autoclaves are unavailable or too expensive. In modern hospitals with disposable needles and knowledge of basic hygiene and barrier nursing techniques, Ebola has never spread on a large scale. In isolated settings such as a quarantined hospital or a remote village, most victims are infected shortly after the first case of infection is present. The quick onset of symptoms from the time the disease becomes contagious in an individual makes it easy to identify sick individuals and limits an individual's ability to spread the disease by traveling. Because bodies of the deceased are still infectious, some doctors had to take measures to properly dispose dead bodies in a safe manner despite local traditional burial rituals.
Prevalence
For more about specific outbreaks and their descriptions, see List of Ebola outbreaks.Outbreaks of Ebola, with the exception of Reston ebolavirus, have mainly been restricted to Africa. The virus often consumes the population, governments and individuals quickly respond to quarantine the area, and the lack of roads and transportation—helps to contain the outbreak.
- Zaire virus first emerged in an outbreak among human populations in 1976 in Zaire (now Democratic Republic of the Congo) with no further recognized cases until 1994. Since then it has occurred again in the Democratic Republic of the Congo, Republic of the Congo, and Gabon. There have been two contained cases in South Africa.
- Sudan ebolavirus emerged in a simultaneous outbreak with the Zaire virus in 1976 in Sudan. It appeared again in another outbreak in 1979. No further cases were recognized until a 2000 outbreak in Uganda and 2004 outbreak in Sudan. There has been one confirmed accidental incidence in 1976 in England.
- Reston ebolavirus was first recognized among monkeys in 1989 in the Reston, Virginia and again in Alice, Texas in the United States, both were traced to the Philippines. In 1994 it was recognized in cases among monkeys in an import facility in Italy. In 2008 cases of infection among pigs were recognized in the Philippines.
- Ivory Coast ebolavirus was first recognized in 1994 after a scientist became ill after conducting an autopsy on a wild chimpanzee in the Tai Forest, Côte d'Ivoire.
- Bundibugyo ebolavirus was first recognized in 2007 in an outbreak in Bundibugyo District, Uganda.
History
Emergence
For more about the outbreak in Virginia, see Reston ebolavirus.Ebolavirus first emerged in 1976 in outbreaks of Ebola hemorrhagic fever in Zaire and Sudan. The strain of Ebola that broke out in Zaire has one of the highest case fatality rates of any human pathogenic virus, roughly 90%, with case-fatality rates at 88% in 1976, 59% in 1994, 81% in 1995, 73% in 1996, 80% in 2001-2002, and 90% in 2003. The strain that broke out later in Sudan has a case fatality rate of around 50%. The virus is believed to be transmitted to humans via contact with an infected animal host. The virus is then transmitted to other people that come into contact with blood and bodily fluids of the infected person, and by human contact with contaminated medical equipment such as needles. Both of these infectious mechanisms will occur in clinical (nosocomial) and non-clinical situations. Due to the high fatality rate, the rapidity of demise, and the often remote areas where infections occur, the potential for widespread epidemic outbreaks is considered low.
Proceedings of an International Colloquium on Ebola Virus Infection and Other Hemorrhagic Fevers were held in Antwerp, Belgium on December 6 through December 8 in 1977.
While investigating an outbreak of Simian hemorrhagic fever (SHFV) in November 1989, an electron microscopist from USAMRIID discovered filoviruses similar in appearance to Ebola in tissue samples taken from Crab-eating Macaque imported from the Philippines to Hazleton Laboratories Reston, Virginia. Due to the lethality of the suspected and previously obscure virus, the investigation quickly attracted attention.
Blood samples were taken from 178 animal handlers during the incident. Of those, six animal handlers eventually seroconverted. When the handlers failed to become ill, the CDC concluded that the virus had a very low pathogenicity to humans.
Philippines and the United States had no previous cases of infection, and upon further isolation it was concluded to be another species of Ebola or a new filovirus of Asian origin, and named Reston ebolavirus (REBOV) after the location of the incident.
Recent cases
In 1992, members of Japan's Aum Shinrikyo cult considered using Ebola as a terror weapon. Their leader, Shoko Asahara, led about forty members to Zaire under the guise of offering medical aid to Ebola victims in a presumed attempt to acquire a virus sample. Because of the virus's high morbidity, it is a potential agent for biological warfare.
Given the lethal nature of Ebola, and, since no approved vaccine or treatment is available, it is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare. The effectiveness as a biological weapon is compromised by its rapid lethality as patients quickly die off before they are capable of effectively spreading the contagion.
The attention gathered from the outbreak in Reston prompted an increase in public interest, leading to the publication of numerous fictional works.
The BBC reports in a study that frequent outbreaks of ebola may have resulted in the deaths of 5,000 gorillas.
As of August 30, 2007, 103 people (100 adults and three children) were infected by a suspected hemorrhagic fever outbreak in the village of Kampungu, Democratic Republic of the Congo. The outbreak started after the funerals of two village chiefs, and 217 people in four villages fell ill. The World Health Organization sent a team to take blood samples for analysis and confirmed that many of the cases are the result of Ebolavirus. The Congo's last major Ebola epidemic killed 245 people in 1995 in Kikwit, about 200 miles from the source of the August 2007 outbreak.
On November 30, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus which is now tentatively named Bundibugyo. The epidemic came to an official end on February 20, 2008. While it lasted, 149 cases of this new strain were reported, and 37 of those led to deaths.
An International Symposium to explore the environment and filovirus, cell system and filovirus interaction, and filovirus treatment and prevention was held at Centre Culturel Français, Libreville, Gabon during March 2008. The virus appeared in southern Western Kasai Province on November 27, 2008, and blood and stool samples were sent to laboratories in Gabon and South Africa for identification.
On December 25, 2008, a mysterious disease that had killed eleven and infected twenty-one people in southern Democratic Republic of Congo was identified as the Ebola virus. Doctors Without Borders reported 11 deaths as of Monday 29 December 2008 in the Western Kasai province of the Democratic Republic of Congo, stating that a further 24 cases were being treated. In January 2009, Angola closed down part of their border with DRC to prevent the spread of the outbreak.
On March 12, 2009, an unidentified 45-year-old female scientist from Germany accidentally pricked her finger with a needle used to inject Ebola into lab mice. She was given an experimental vaccine never before used on humans. Since the peak period for an outbreak during the 21-day Ebola incubation period has passed as of April 2, 2009, she has been declared healthy and safe. It remains unclear whether or not she was ever actually infected with the virus.
In other animals
Outbreaks of Ebola among human populations generally result from handling infected wild animal carcasses. Declines in animal populations generally precede outbreaks among human populations. These have led to in 2003 surveillance of animal populations in order to predict and prevent Ebola outbreaks.
Recovered carcasses from gorillas contain multiple Ebola strains, which suggest multiple introductions of the virus. Bodies decompose quickly and carcasses are not infectious after three to four days. Contact between gorilla groups is rare. This suggests transmission among gorilla groups unlikely and that outbreaks result from transmission between viral reservoir and animal populations.
Outbreaks of Ebola have been responsible for an 88% decline in observed chimpanzee populations since 2003. Transmission among chimpanzees through meat consumption constitute a significant 5.2 (1.3-21.1 with 95% confidence) relative risk factor, while contact between individuals such as touching dead bodies and grooming do not.
Reston ebolavirus, which has not had a previous outbreak in Africa and is non-pathogenic in humans, has recently been recognized among swine populations in the Philippines; this discovery suggests that the virus has been circulating since and possibly before the initial discovery of Reston ebolavirus in 1989 among monkeys.
References
- Bardi, Jason Socrates (2002). "Death Called a River". Scripps Research Institute. 2 (1). Retrieved 2006-12-08.
- Netesov, SV; Feldmann, H; Jahrling, PB; Kiley, MP; Klenk, H-D; Sanchez, A (2004-04-24). "Filoviridae". International Committee on Taxonomy of Viruses. Retrieved 2009-10-04.
- Büchen-Osmond, Cornelia (2006-04-25). "ICTVdB Virus Description - 01.025.0.02. Ebolavirus". International Committee on Taxonomy of Viruses. Retrieved 2009-06-02.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9254917, please use {{cite journal}} with
|pmid=9254917instead. - Isaacson, M; Sureau, P; Courteille, G; Pattyn, SR;. "Clinical Aspects of Ebola Virus Disease at the Ngaliema Hospital, Kinshasa, Zaire, 1976". Retrieved 2009-07-08.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Special Pathogens Branch CDC (2008-01-14). "Known Cases and Outbreaks of Ebola Hemorrhagic Fever". Center for Disease Control and Prevention. Retrieved 2008-08-02.
- McNeil Jr, Donald G. (2009-01-24). "Pig-to-Human Ebola Case Suspected in Philippines". New York Times. Retrieved 2009-01-26.
- McCormick & Fisher-Hoch 1999, p. 300
- Waterman, Tara (1999). Ebola Cote D'Ivoire Outbreaks. Stanford University. Retrieved 2009-05-30.
- "End of Ebola outbreak in Uganda" (Press release). World Health Organization. 2008-02-20.
- Cocks, Tim (2007-12-11). "Uganda confirms 113 suspected Ebola cases". Reuters. Retrieved 2008-02-25.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 14718087, please use {{cite journal}} with
|pmid=14718087instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1371/journal.pmed.0010059, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1371/journal.pmed.0010059instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9893380, please use {{cite journal}} with
|pmid=9893380instead. - Zaki, S. R.; Kilmarx, P. (1997). "Ebola Virus Hemorrhagic Fever". Pathology of Emerging Infections. Washington, DC: American Society for Microbiology.
- Klenk & Feldmann 2004, p. 2
- Klenk & Feldmann 2004, p. 13
- Klenk & Feldmann 2004, pp. 33–35
- Klenk & Feldmann 2004, pp. 28
- ^ Biomarker Database. Ebola virus. Korea National Institute of Health. Retrieved 2009-05-31.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8219816, please use {{cite journal}} with
|pmid=8219816instead. - Klenk & Feldmann 2004, p. 9
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1128/JVI.77.18.9733-9737.2003, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1128/JVI.77.18.9733-9737.2003instead. - McCormick & Fisher-Hoch 1999, pp. 302–303
- Ebola and Marburg haemorrhagic fever - Factsheet. European Centre for Disease Prevention and Control. 2008-07-10. Retrieved 2009-05-31.
{{cite book}}: Check date values in:|year=/|date=mismatch (help) - Centers for Disease Control and Prevention and World Health Organization (1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Atlanta, Georgia, USA: Centers for Disease Control and Prevention. Retrieved 2009-05-31.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nature01876, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nature01876instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nm1258, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nm1258instead. - Oplinger, Anne A. (2003-11-18). NIAID Ebola vaccine enters human trial. Bio-Medicine.
- "Ebola/Marburg Vaccine Development" (Press release). National Institute of Allergy and Infectious Diseases. 2008-09-15.
- Linden, Caree Vander (2006-01-13). Gene-Specific Ebola Therapies Protect Nonhuman Primates from Lethal Disease (New Release). U.S. Army Medical Research Institute of Infectious Diseases. Retrieved 2009-05-31.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1128/AAC.00936-08, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1128/AAC.00936-08instead. - ^ "Drug shields lab monkeys from deadly Ebola virus". AFP. 29 May 2010. Retrieved 30 May 2010.
- Knox, Richard (28 May 2010). "New Ebola Drug 100 Percent Effective In Monkeys". National Public Radio. Retrieved 30 May 2010.
- Skelton, Chad (29 May 2010). "Potential Ebola cure uses made-in-B. C. technology". Vancouver Sun. Retrieved 30 May 2010.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15752448, please use {{cite journal}} with
|pmid=15752448instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.biocel.2005.02.018, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.biocel.2005.02.018instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.micinf.2005.04.006, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.micinf.2005.04.006instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S1286-4579(99)00242-7, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S1286-4579(99)00242-7instead. - "Fruit bats may carry Ebola virus". BBC News. 2005-12-11. Retrieved 2008-02-25.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8969248, please use {{cite journal}} with
|pmid=8969248instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/438575a, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/438575ainstead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17940947, please use {{cite journal}} with
|pmid=17940947instead. - Lubroth, Juan. "Ebola-Reston Virus in Pigs: Disease situation in swine in the Philippines". Food and Agriculture Organization of the United Nations. Retrieved 2009-09-27.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17848072, please use {{cite journal}} with
|pmid=17848072instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15078595, please use {{cite journal}} with
|pmid=15078595instead. - Questions and Answers about Ebola Hemorrhagic Fever. Centers for Disease Control and Prevention. 2009-03-25. Retrieved 2009-05-31.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8551825, please use {{cite journal}} with
|pmid=8551825instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8712894, please use {{cite journal}} with
|pmid=8712894instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7547435, please use {{cite journal}} with
|pmid=7547435instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15588056, please use {{cite journal}} with
|pmid=15588056instead. - Harden, Blaine (2001-02-18). "Dr. Matthew's Passion". New York Times Magazine. Retrieved 2008-02-25.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7787519, please use {{cite journal}} with
|pmid=7787519instead. - ^ King, John W (April 2, 2008). "Ebola Virus". eMedicine. WebMd. Retrieved 2008-10-06.
- http://methoo.com/ebola/
- Pattyn 1978, p. 3
- McCormick & Fisher-Hoch 1999, pp. 277–279
- Waterman, Tara (1999). Ebola Reston Outbreaks. Stanford University. Retrieved 2008-08-02.
- McCormick & Fisher-Hoch 1999, pp. 298–299
- Monterey Institute for International Studies (2001). Chronology of Aum Shinrikyo's CBW Activities (PDF). James Martin Center for Nonproliferation Studies.
- Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15207310, please use {{cite journal}} with
|pmid=15207310instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1001/jama.287.18.2391, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1001/jama.287.18.2391instead. - Ebola 'kills over 5,000 gorillas'. BBC. 2006-12-08. Retrieved 2009-05-31.
- "Ebola Outbreak Confirmed in Congo". NewScientist.com. 2007-09-11. Retrieved 2008-02-25.
- Ebola outbreak in Congo. CDC news. 2007-09-12. Retrieved 2009-05-31.
- "Mystery DR Congo fever kills 100". BBC News. 2007-08-31. Retrieved 2008-02-25.
- "Uganda: Deadly Ebola Outbreak Confirmed - UN". UN News Service. 2007-11-30. Retrieved 2008-02-25.
- The IV International Symposium on Filoviruses. l'Institut de recherche pour le développement (IRD). Retrieved 2009-0-31.
{{cite book}}: Check date values in:|accessdate=(help) - World Health Organization (2008-12-27). RD Congo : Fièvre hémorragique à virus Ebola au Kasaï Occidental, Rapport de situation No 1 des 26 & 27 décembre 2008 (in French). Relief Web. Retrieved 2009-06-02.
- Ebola epidemic kills nine in central DR Congo: report. Agence France-Presse. 2008-12-25. Retrieved 2009-05-30.
- Ebola alert shuts Angolan border. BBC. 2009-01-06. Retrieved 2009-05-31.
- Eddyn, Melissan (2009-03-27). "Scientist Injects Self With Ebola". Associated Press. Retrieved 2009-05-02.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.1092528, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.1092528instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9988175, please use {{cite journal}} with
|pmid=9988175instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19590002, please use {{cite journal}} with
|pmid=19590002instead.
Bibliography
- Klenk, Hans-Dieter (1999). Marburg and Ebola Viruses (Current Topics in Microbiology and Immunology). Berlin, Germany: Springer-Verlag Telos. ISBN 978-3540647294.
{{cite book}}: Unknown parameter|month=ignored (help)CS1 maint: ref duplicates default (link) - Klenk, Hans-Dieter; Feldmann, Heinz (2004). Ebola and Marburg viruses: molecular and cellular biology (Limited preview). Wymondham, Norfolk, UK: Horizon Bioscience. ISBN 978-0954523237.
{{cite book}}: CS1 maint: ref duplicates default (link) - Kuhn, Jens H. (2008). Filoviruses - A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. Archives of Virology Supplement, vol. 20 (Limited preview). Vienna, Austria: SpringerWienNewYork. ISBN 978-3211206706.
{{cite book}}: CS1 maint: ref duplicates default (link) - McCormick, Joseph; Fisher-Hoch, Susan (1999) . Level 4: Virus Hunters of the CDC (Limited preview). Horvitz, Leslie Alan ("Updated edition" 3rd ed.). Barnes & Noble. ISBN 9780760712085.
{{cite book}}: Unknown parameter|origmonth=ignored (help)CS1 maint: ref duplicates default (link) - Pattyn, S. R. (1978). Ebola Virus Haemorrhagic Fever (Full free text) (1st ed.). Amsterdam, Netherlands: Elsevier/North-Holland Biomedical Press. ISBN 0-444-80060-3.
{{cite book}}: CS1 maint: ref duplicates default (link) - Ryabchikova, Elena I.; Price, Barbara B. (2004). Ebola and Marburg Viruses - A View of Infection Using Electron Microscopy. Columbus, Ohio, USA: Battelle Press. ISBN 978-1574771312.
{{cite book}}: CS1 maint: ref duplicates default (link)
External links
- ViralZone: Ebola-like viruses — Virological repository from the Swiss Institute of Bioinformatics
- CDC: Ebola Hemorrhagic Fever — Centers for Disease Control and Prevention, Special Pathogens Branch
- WHO: Ebola haemorrhagic fever — World Health Organization, Global Alert and Response