This is an old revision of this page, as edited by Spinach Dip (talk | contribs) at 05:32, 25 August 2011 (formatting). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 05:32, 25 August 2011 by Spinach Dip (talk | contribs) (formatting)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Diphosphorus | |||
| Systematic IUPAC name Diphosphyne | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| Gmelin Reference | 1400241 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | P2 | ||
| Molar mass | 61.947523996 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Diphosphorus, P2, is the diatomic form of phosphorus. Unlike its nitrogen group neighbor nitrogen which forms a stable N2 molecule with a nitrogen to nitrogen triple bond, phosphorus prefers a tetrahedral form P4 because P-P pi-bonds are high in energy. Diphosphorus is therefore very reactive with a bond dissociation energy (117 kcal/mol or 490 kJ/mol) half that of dinitrogen.
Traditionally diphosphorus can be generated by heating white phosphorus at 1100 kelvins. Nevertheless, some advancements were obtained in generating the diatomic molecule in homogeneous solution, under normal conditions with the use by some transition metal complexes (based on for example tungsten and niobium).
The molecule attracted attention in 2006 when a new method for its synthesis at milder temperatures emerged.
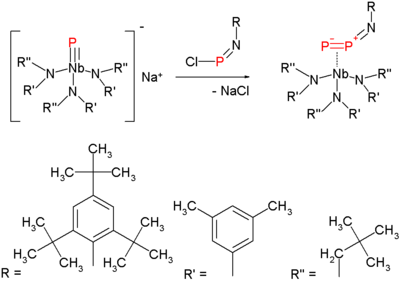
This method is a variation on nitrogen expulsion in azides with formation of a nitrene. The synthesis of the diphosphorus precursor consists of reacting a terminal niobium phosphide with a chloroiminophosphane:
Heating this compound at 50 °C in 1,3-cyclohexadiene serving as a solvent and as a trapping reagent, expulses diphosphorus which reactive as it is forms a double Diels-Alder adduct and the niobium imido compound:
The same imido compound also forms when the thermolysis is performed in toluene but then the fate of diphosphorus is unknown.
P2 has been suggested to form as an intermediate in the photolysis of P4 and in the presence of 2,3-dimethylbutadiene the diphosphane is again formed To date, no direct evidence of P2 formation via P4 photolysis exists.
References
- "Diphosphorus (CHEBI:33472)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- Russell, C. A. (2010), PP, a Laboratory Reagent?. Angewandte Chemie International Edition, 49: 9572–9573. doi:10.1002/anie.201006243
- Triple-Bond Reactivity of Diphosphorus Molecules Nicholas A. Piro, Joshua S. Figueroa, Jessica T. McKellar, Christopher C. Cummins Science 1 September 2006:Vol. 313. no. 5791, pp. 1276–1279 10.1126/science.1129630
- http://www.sciencemag.org/cgi/reprint/313/5791/1276.pdf
- Triple-Bond Reactivity of Diphosphorus Molecules Nicholas A. Piro, Joshua S. Figueroa, Jessica T. McKellar, Christopher C. Cummins Science 1 September 2006:Vol. 313. no. 5791, pp. 1276 - 1279 doi:10.1126/science.1129630 PMID 16946068
- Optische und photochemische versuche mit phosphor G. Rathenau Physica Volume 4, Issue 6, June 1937, Pages 503-514 doi:10.1016/S0031-8914(37)80084-1
- Tofan, D. and Cummins, C. C. (2010), Photochemical Incorporation of Diphosphorus Units into Organic Molecules. Angewandte Chemie International Edition, 49: 7516–7518. doi: 10.1002/anie.201004385
External links
- Ron Dagani, "A Mild Route To P2, Chemical & Engineering News September 4, 2006 Link