This is an old revision of this page, as edited by Codename:Iceman (talk | contribs) at 23:39, 6 November 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:39, 6 November 2011 by Codename:Iceman (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. Please help improve this article by introducing more precise citations. (November 2011) (Learn how and when to remove this message) |
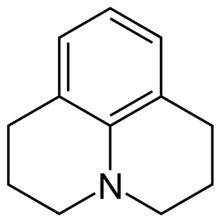
| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.006.851 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C12H15N |
| Molar mass | 173.26 g/mol |
| Density | 1.003 g/ml |
| Melting point | 35 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Julolidine is a heterocyclic aromatic organic compound. It has the formula C12H15N.
Synthesis
Synthesized of Julolidine was first reported by G. Pinkus in 1892.
Applications
This compound and its derivatives have found recent interest as photoconductive materials, chemiluminescence substances, chromogenic substrates in analytical redox reactions, dye intermediates, potential antidepressants and tranquilizers, nonlinear optical materials, high sensitivity photopolymerizable materials, and for improving color stability in photography.
References
- Pinkus, G. Ueber die Einwirkung von Trimethylenchlorbromid auf einige aromatische Amine und Amide. Berichte der deutschen chemischen Gesellschaft 1892, 25 (2), 2798–2806.
- Katritzky, A.; Rachwal, B.; Rachwal, S. Convenient Synthesis of Julolidines Using Benzotriazole Methodology. J. Org. Chem. Rachwal 1996, 61, 3117–3126.
| This article has not been added to any content categories. Please help out by adding categories to it so that it can be listed with similar articles. (November 2011) |