This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 12:21, 21 October 2011 (Updating {{drugbox}} (changes to verified and watched fields - updated 'ChEBI_Ref', 'KEGG_Ref', 'CAS_number_Ref') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:21, 21 October 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (changes to verified and watched fields - updated 'ChEBI_Ref', 'KEGG_Ref', 'CAS_number_Ref') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound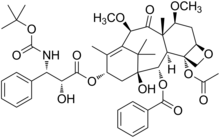 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611009 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.205.741 |
| Chemical and physical data | |
| Formula | C45H57NO14 |
| Molar mass | 835.93 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Cabazitaxel (previously XRP-6258, trade name Jevtana) is a semi-synthetic derivative of a natural taxoid. It was developed by Sanofi-Aventis and was approved by the U.S. Food and Drug Administration (FDA) for the treatment of hormone-refractory prostate cancer on June 17, 2010. It is a microtubule inhibitor.
Cabazitaxel in combination with prednisone is a treatment option for hormone-refractory prostate cancer following or during docetaxel-based treatment.
Clinical trials
In a phase III trial with 755 men for the treatment of hormone-refractory prostate cancer, median survival was 15.1 months for patients receiving cabazitaxel versus 12.7 months for patients receiving mitoxantrone. Cabazitaxel was associated with more grade 3-4 neutropenia (81.7%) than mitoxantrone (58%).
References
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- http://www.cancer.gov/drugdictionary/?CdrID=534131
- "Jevtana (cabazitaxel) Injection Approved by U.S. FDA After Priority Review" (Press release). sanofi-aventis. 2010-06-17. Retrieved June 17, 2010.
- "Cabazitaxel Effective for Hormone Refractory Prostate Cancer After Failure of Taxotere".
External links
- Cabazitaxel - Official web site of manufacturer.
- Cabazitaxel Prescribing Information - Official prescribing information.
- U.S. National Library of Medicine: Drug Information Portal - Cabazitaxel
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |