This is an old revision of this page, as edited by DV8 2XL (talk | contribs) at 20:33, 17 April 2006 (Your delusional misinterpertations of the literature are not supported by your sources, James Reverting away nonsence.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 20:33, 17 April 2006 by DV8 2XL (talk | contribs) (Your delusional misinterpertations of the literature are not supported by your sources, James Reverting away nonsence.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| Uranium trioxide | |
|---|---|

|
solid γ-UO3 (gamma polymorph) oxygen diameters sharply reduced for visibility |
| General | |
| Systematic name | Uranium trioxide Uranium(VI) oxide |
| Other names | Uranyl oxide Uranic oxide |
| Molecular formula | UO3 ) |
| CAS number | |
| Properties | |
| Molar mass | 286.2873 g/mol Commercial samples may have undergone isotope fractionation, and their molecular mass may be significantly different |
| Density and phase | 5.5 – 8.7 g/cm |
| Solubility (water) | Partially soluble |
| Solubility (dog lung fluid) | < 5 days (Morrow, 1972) |
| Melting point | ~ 200 – 650 °C decomp. (s) |
| Structure | |
| Molecular shape | T-shape |
| Coordination geometry |
γ-UO3: |
| Crystal structure | I41/amd (γ-UO3) |
| Hazards | |
| MSDS | UO3-MSDS |
| Main hazards | highly toxic: teratogen, immunotoxin, neurotoxin, genotoxin, nephrotoxin |
| Flash point | inflamable |
| Related compounds | |
| Other anions | Uranyl nitrate |
| Other cations | Chromium trioxide |
| Related compounds | Uranium dioxide Triuranium octaoxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The toxic, teratogenic, and radioactive solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.
Production and use
There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium entrichment.
- U3O8 can be oxidized at 500°C with oxygen.
- Uranyl nitrate, (UO2(NO3)2·6H2O) can be heated to yield UO3. This occurs during the reprocessing of nuclear fuel. Fuel rods are dissolved in HNO3 to separate uranyl nitrate from plutonium and the fission products (the PUREX method). The pure uranyl nitrate is converted to solid UO3 by heating at 400 °C. After reduction with hydrogen (with with other inert gas present) to uranium dioxide, the uranium can be used in new MOX fuel rods.
- Ammonium diuranate or sodium diuranate (Na2U2O7·6H2O) may be decomposed. Sodium diuranate, also known as yellowcake, is converted to uranium trioxide in the enrichment of uranium. Uranium dioxide and uranium tetrafluoride are intermediates in the process which ends in uranium hexafluoride.
Uranium trioxide is shipped between processing facilities in the form of a gel.
Cameco Corporation, which operates at the world's largest uranium refinery at Blind River, Ontario, produces high-purity uranium trioxide.
Health and safety hazards
Like all hexavalent uranium compounds (also called uranium(VI) compounds), UO3 is hazardous by inhalation, ingestion, and through skin contact. It is a poisonous, radioactive substance, which may cause shortness of breath, coughing, acute arterial lesions, and changes in the chromosomes of white blood cells and gonads leading to congenital malformations if inhaled.
Chemistry and structure
Solid state
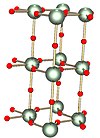

The only well characterized binary trioxide of any actinide is UO3, of which several polymorphs are known. Solid UO3 loses O2 on heating to give green-colored U3O8: reports of the decomposition temperature in air vary from 200–650 °C. Heating at 700 °C under H2 gives dark brown uranium dioxide (UO2), which is used in MOX nuclear fuel rods.
Uranium trioxide reacts at 400 °C with freon-12 to form chlorine, phosgene, carbon dioxide and uranium(IV) fluoride. The freon-12 can be replaced with freon-11 which forms carbon tetrachloride instead of carbon dioxide. This is a case of a hard perhalogenated freon which is normally considered to be inert being converted chemically at a moderate temperture.
2 CF2Cl2 + UO3 → UF4 + CO2 + COCl2 + Cl2
4 CF2Cl2 + UO3 → UF4 + 3COCl2 + CCl4 + Cl2
Uranium trioxide can be dissolved in a mixture of tributyl phosphate and thenoyltrifluoroacetone in supercritical carbon dioxide, ultrasound was employed during the dissolution.
The most frequently encountered polymorph is γ-UO3, whose x-ray structure has been solved from powder diffraction data. The compound crystallizes in the space group I41/amd with two uranium atoms in the asymmetric unit. Both are surrounded by somewhat distorted octahedra of oxygen atoms. One uranium atom has two closer and four more distant oxygen atoms whereas the other has four close and two more distant oxygen atoms as neighbors. Thus it is not incorrect to describe the structure as , that is uranyl uranate. .
High presure solid forms exist. Gmelin Handbuch (1982) U-C1, 129-135.
-
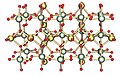 The γ (gamma) form, with the different uranium environments in green and yellow
The γ (gamma) form, with the different uranium environments in green and yellow
-
 The environment of the uranium atoms shown as yellow in the gamma form
The environment of the uranium atoms shown as yellow in the gamma form
-
 The chains of U2O2 rings in the gamma form in layers, alternate layers running at 90 degrees to each other. These chains are shown as containing the yellow uranium atoms, in a octahedral environment which are distorted towards square planar by an elongation of the axial oxygen-uranium bonds.
The chains of U2O2 rings in the gamma form in layers, alternate layers running at 90 degrees to each other. These chains are shown as containing the yellow uranium atoms, in a octahedral environment which are distorted towards square planar by an elongation of the axial oxygen-uranium bonds.
-
 The delta (δ) form was reported by Weller et al. (1988) Polyhedron, 7, 243-244.
The delta (δ) form was reported by Weller et al. (1988) Polyhedron, 7, 243-244.
bond valence parameters
It is possible by bond valence calculations it is possible to estimate how great a contribution a given oxygen atom is making to the assumed valence of uranium. Zachariasen, J. Less Common Met., 1978, 62, 1-7. Lists the parameters to allow such calculations to be done for many of the actinides.
The formula to use is
The sum of the s values is equal to the oxidation state of the metal centre.
For uranium binding to oxygen the constants Ro and B are tabulated in the table below. For each oxidation state use the parameters from the table shown below.
| Oxidation state | Ro | B |
|---|---|---|
| U(VI) | 2.08Å | 0.35 |
| U(V) | 2.10Å | 0.35 |
| U(IV) | 2.13Å | 0.35 |
It is possible to do these calculations on paper or software which does it can be obtained free of charge.
Gas phase
While uranium trioxide is mostly encountered as a solid some work has been done on its vapor, too.
Infrared spectroscopy of molecular UO3 isolated in an argon matrix indicates a T-shaped structure (point group C2v) for the molecule. This is in contrast to the commonly encountered D3h symmetry exhibited by most trioxides. From the force constants the authors deduct the U-O bond lengths to be between 1.76 and 1.79 angstroms (176 to 179 picometers).
At elevated temperatures gaseous UO3 and O2 are in equilibrium with solid U3O8.
- /3 U3O8(s) + /6 O2(g) UO3(g)
With increasing temperature the equilibrium is shifted to the right. This system has been studied at temperatures between 900 and 1500 °C. The vapor pressure of monomeric UO3 is low but appreciable, about 10 mbar (1 mPa) at 980 °C, rising to 10 mbar (10 Pa) at 1400 °C, 0.34 mbar (34 Pa) at 1800 K, 19 mbar (1.9 kPa) at 2000 K, and 81 mbar (8.1 kPa) at 2200 K.
Uranium oxides in ceramics
UO3-based ceramics become green or black when fired in a reducing atmosphere and yellow to orange when fired with oxygen. Orange-coloured Fiestaware is a well-known example of a product with a uranium-based glaze. UO3-has also been used in formulations of enamel, uranium glass, and porcelain.
Prior to 1960, UO3 was used as an agent of crystallization in crystalline coloured glazes. It is possible to determine with a Geiger counter if a glaze or glass was made from UO3.
Related anions and cations
Uranium oxide is amphoteric and reacts as acid and as a base, depending on the conditions.
As an acid:
- UO3 + H2O → UO4 + H
Dissolving uranium oxide in a strong base like sodium hydroxide forms the doubly negatively charged uranate anion (UO4). Uranates tend to agglomerate, forming diuranate, U2O7
As a base:
- UO3 + H2O → UO2 + OH
Dissolving uranium oxide in a strong acid like sulfuric or nitric acid forms the double positive charged uranyl cation. The uranyl nitrate formed (UO2(NO3)2ˑ6H2O) is soluble in ethers, alcohols, ketones and esters; for example, tributylphosphate. This solubilty is used to separate uranium from other elements in nuclear reprocessing, which begins with the dissolution of nuclear fuel rods in nitric acid. The uranyl nitrate is then converted to uranium trioxide by heating.
From nitric acid one obtains uranyl nitrate, trans-UO2(NO3)2·2H2O, consisting of eight-coordinated uranium with two bidentate nitrato ligands and two water ligands as well as the familiar O=U=O core.
See also
Accidental teratogens:
References
- Sheft I, Fried S, Davidson N (1950). "Preparation of Uranium Trioxide". Journal of the American Chemical Society. 72: 2172–2173.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI] - Dell RM, Wheeler V J (1962). "Chemical Reactivity of Uranium Trioxide Part 1.conversion to U3O8, U02 and UF4". Transaction Faraday Society: 1590–1607. DOI
- Morrow, PE, Gibb FR, Beiter HD (1972). "Inhalation studies of uranium trioxide". Health Physics. 23: 273–280.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Abstract - Sutton M, Burastero SR (2004). "Uranium(VI) solubility and speciation in simulated elemental human biological fluids". Chemical Research in Toxicology. 17: 1468–1480. DOI
- Trofimov TI, Samsonov MD, Lee SC, Myasoedov BF, Wai CM (2001). "Dissolution of uranium oxides in supercritical carbon dioxide containing tri-n-butyl phosphate and thenoyltrifluoroacetone". Mendeleev Communications. 11: 129–130.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Booth HS, Krasny-Ergen W,Heath RE (1946). "Uranium Tetrafluoride". Journal of the American Chemical Society. 68: 1969–1970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI - Engmann R, de Wolff PM (1963). "The Crystal Structure of γ-UO3". Acta Crystallographica. 16: 993. DOI
- Wilson WB (1961). "High-Pressure High-Temperature Investigation of the Uranium-Oxygen System". Journal Inorganic Nuclear Chemistry. 19: 212–222. DOI
- Gabelnick SD, Reedy GT, Chasanov MG (1973). "Infrared spectra of matrix-isolated uranium oxide species. II: Spectral interpretation and structure of UO3". 59: 6397.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link)
- Ackermann RJ, Thorn RJ, Alexander C, Tetenbaum M (1960). "Free Energies of Formation of Gaseous Uranium, Molybdenum, and Tungsten Trioxides". Journal of Physical Chemistry. 64: 350–355.
{{cite journal}}: CS1 maint: multiple names: authors list (link) DOI page350 351 352 353 354 355 - Ackermann RJ, Gilles PW, Thorn RJ (1956). Journal of Chemical Physics. 25: 1089.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - {{cite journal | author= Alexander CA | title= Volatilization of urania under strongly oxidizing conditions | journal= Journal of Nuclear Materials | year= 2005 | volum

 UO3(g)
UO3(g)