This is an old revision of this page, as edited by Edwyth (talk | contribs) at 08:26, 5 April 2022 (→Vaccination program). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 08:26, 5 April 2022 by Edwyth (talk | contribs) (→Vaccination program)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Plan to immunize against COVID-19
 | |
| Date | December 14, 2020 (2020-12-14) – present |
|---|---|
| Location | Compact of Free Association: |
| Cause | COVID-19 pandemic in the United States |
| Organized by | Center for Disease Control and Prevention |
| Participants | 241,520,561 people have received at least one dose administered of Pfizer–BioNTech, Moderna or Janssen (December 23, 2021) 204,740,321 people have been fully vaccinated (both doses of Pfizer–BioNTech or Moderna, or one dose of Janssen) |
| Outcome | 73% of the United States population has received at least one dose of a vaccine
62% of the United States population is fully vaccinated |
| Website | COVID-19 Vaccine: CDC COVID-19 Vaccinations in the United States |

The COVID-19 vaccination campaign in the United States is an ongoing mass immunization campaign for the COVID-19 pandemic in the United States. The Food and Drug Administration (FDA) first granted emergency use authorization to the Pfizer–BioNTech vaccine on December 10, 2020; mass vaccinations began on December 14, 2020. The Moderna vaccine was granted emergency use authorization on December 17, 2020, and the Janssen (Johnson & Johnson) vaccine was granted emergency use authorization on February 27, 2021. By April 19, 2021, all U.S. states had opened vaccine eligibility to residents aged 16 and over. On May 10, 2021, the FDA approved the Pfizer-BioNTech vaccine for adolescents aged 12 to 15. On August 23, 2021, the FDA granted full approval to the Pfizer–BioNTech vaccine for individuals aged 16 and over.
The U.S. government first initiated the campaign under the presidency of Donald Trump with Operation Warp Speed, a public–private partnership to expedite the development and manufacturing of COVID-19 vaccines. Joe Biden became the new President of the United States on January 20, 2021. Biden began his term with an immediate goal of administering 100 million vaccine doses within his first hundred days in office, signing an executive order which included increasing supplies for vaccination. This goal was met on March 19, 2021. On March 25, 2021, he announced he would increase the goal to 200 million within his first 100 days in office. This goal was eventually reached on April 21, 2021.
By July 4, 2021, 67% of the United States' adult population had received at least one dose, just short of a goal of 70%. This goal was eventually met on August 2, 2021. While vaccines have helped significantly reduce the number of new COVID-19 infections nationwide, states with below-average vaccination rates began to see increasing numbers of cases credited to the highly infectious Delta variant by July 2021, which led to an increased push by organizations and companies to begin imposing de facto mandates for their employees be vaccinated for COVID-19.
On September 9, 2021, President Biden announced plans by the federal government to use executive orders and emergency temporary standards enforced by OSHA to mandate the vaccination of all federal branch employees, and require that all companies with more than 100 employees regularly test all employees who are not yet fully vaccinated for COVID-19. On January 26, 2022, OSHA completely withdrew the vaccine mandate for companies with more than 100 employees due to a ruling from The Supreme Court of the United States that blocked the mandate.
As of November 2021, according to The Commonwealth Fund, COVID-19 vaccination in the United States has prevented an additional 1.1 million deaths, an additional 10.3 million hospitalizations, and an additional 35.9 million infections from COVID-19.
Vaccination program
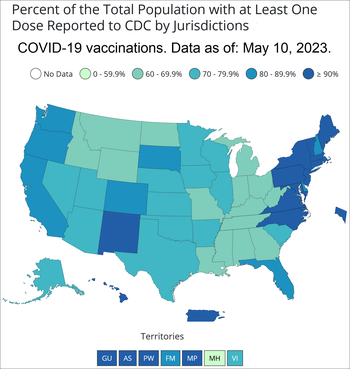
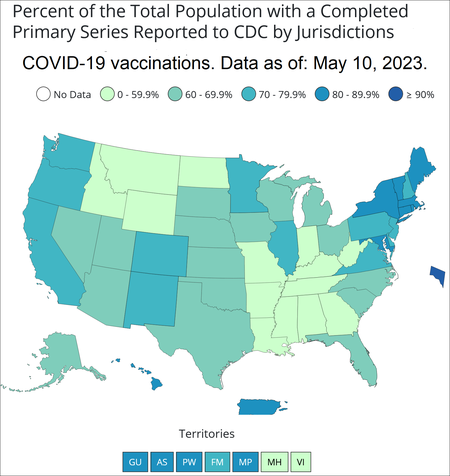
The map below is for the percent of people fully vaccinated for COVID-19 by state. Right now, two of the three COVID-19 vaccines used in the U.S. require two shots to be fully vaccinated. The other vaccine requires only one shot. Booster doses are recommended for people age 12 and older. Additional shots are recommended in some cases.
| Graphs are unavailable due to technical issues. Updates on reimplementing the Graph extension, which will be known as the Chart extension, can be found on Phabricator and on MediaWiki.org. |

Total number of people who have received vaccinations in the United States as of March 5, 2022
Unvaccinated population: ~77.9 million people (23.48%) Population who have received only one dose of a two-dose vaccine: 37,922,939 (11.42%) Population who are fully vaccinated, but haven't received an additional booster shot: 121,005,690 (36.45%) Population who are fully vaccinated and received an additional booster shot: 95,073,718 (28.64%)Vaccines administered per pharmaceutical company as of February 10, 2022
Pfizer–BioNTech (320,648,278) (58.78%) Moderna (206,059,690) (37.78%) Johnson & Johnson (18,283,801) (3.35%) Not Identified (485,351) (0.09%)Vaccines were distributed to states on a population basis, with the vaccine rollouts being administered by each individual U.S. state. The Center for Disease Control and Prevention suggested that hospital workers and nursing home personnel be the first individuals vaccinated. The subsequent phases of the rollout are determined by each individual state agency.
The CDC has issued cards that can be used to track vaccine progress and serve as a proof of vaccination when requested (such as during international travel). States such as California and New York have offered a digital immunity passport accessible via a mobile app.
Eligibility of non-citizens
On February 1, 2021, the Department of Homeland Security said it "fully support equal access to the COVID-19 vaccines and vaccine distribution sites for undocumented immigrants" and that related federal agencies "will not conduct enforcement operations at or near vaccine distribution sites or clinics".
States may have intended that vaccines be prioritized for their residents ahead of tourists, but there was some difficulty communicating and enforcing this. Some American adults have no driver's license, and the United States does not automatically provide each citizen with identity documentation in a centralized system. Furthermore, when people did not show up for their vaccine appointments, many clinics vaccinated anyone else who happened to show up so that the doses would not be wasted. As a result, some tourists as well as undocumented immigrants were vaccinated.
As of early February 2021, states including Florida, California, New York, and Texas were specifically trying to restrict "vaccine tourism": brief visits to the U.S. with the primary intention of obtaining a vaccine. However, contrary to rumors that spread on social media, the United States did not have a policy of cancelling visas or imposing fines on tourists who sought vaccination. Diplomats pointed out that the B1/B2 tourist visa allows people to seek medical treatment while within the United States, even if they do not turn out to be eligible for the COVID-19 vaccine.
As of May 13, 2021, according to the Colombian newspaper El Tiempo, the following U.S. states were not requiring foreigners to present proof of residency to receive the vaccine: Alabama, Arizona, California, Colorado, Florida, Iowa, Louisiana, Maryland, Michigan, Minnesota, Missouri, Montana, Nevada, New Hampshire, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and Wyoming. Thousands of Latin Americans were booking travel to the United States and being vaccinated in the country. Vaccination centers in some U.S. states were accepting foreign passports as valid identification. Travel agencies were advertising "vaccination tourism," and the U.S. embassy in Peru, for example, advised that travelers to the United States could seek vaccination. The governor of Alaska announced that, as of June 1, any international visitor over age 12 could be vaccinated in Alaska and vaccines would be available at airports for convenience.
Background
From early 2020, more than seventy companies worldwide (with five or six operating primarily in the U.S.) began vaccine research. 28 different vaccines are being worked on and developed using the research provided by those companies. The global competition had national security implications for various countries.
In preparation for large-scale production, Congress set aside more than $3.5 billion for this purpose as part of the CARES Act. Among the labs working on a vaccine is the Walter Reed Army Institute of Research, which has previously studied other infectious diseases, such as HIV/AIDS, ebola, and MERS. By March 18, tests had begun with dozens of volunteers in Seattle, sponsored by the U.S. government, and similar safety trials were planned for other potential vaccines. Bill Gates, whose foundation shifted its focus nearly entirely to the pandemic, anticipated in early 2020 that a vaccine could be ready by early 2021.
On August 5, 2020, the United States agreed to pay Johnson & Johnson more than $1 billion to create a hundred million doses of COVID-19 vaccine. The deal gave the U.S. an option to order an additional two hundred million. The doses were supposed to be provided for free to Americans if they are used in a COVID-19 vaccination campaign.
On August 31, 2020, the Centers for Disease Control and Prevention (CDC) released their outline for how the COVID-19 vaccine would be administered and distributed across the entire country.

BIO, a trade group of all COVID-19 vaccine makers except AstraZeneca, tried to persuade Secretary Azar to publish strict FDA guidelines that would help ensure the safety and public uptake of the vaccine. Politics impacted scientific practice, however, when chief of staff Mark Meadows blocked the FDA when it was realized that the timing of the provisions would make it impossible for a vaccine to be authorized before the November election. Ultimately, the guidelines emerged from the Office of Management and Budget and were published on the FDA website.
As of October 2020, 44 were in clinical trials on humans, and 91 pre-clinical vaccines were being tested on animals. Most of these trials were underway.
On November 20, 2020, the Pfizer–BioNTech partnership submitted a request for emergency use authorization (EUA) to the Food and Drug Administration (FDA), and the FDA announced that its Vaccines and Related Biological Products Advisory Committee (VRBPAC) would review the request. An EUA is a mechanism under the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 that permits products not yet fully approved by the FDA to be used as part of a current state of emergency.
On December 11, the FDA granted emergency use authorization for the Pfizer–BioNTech vaccine. An initial shipment of 2.9 million doses were scheduled to be distributed rapidly, and Pfizer promised to continue supplying the rest of the hundred million doses through March 2021. Pfizer had adequate stocks available and began this distribution on December 17, 2020, but the federal government reduced the amount Pfizer was allowed to distribute.
On December 18, 2020, the FDA granted the Moderna vaccine emergency use authorization, which Moderna had requested on November 30, 2020. The U.S. planned to rapidly distribute 5.9 million doses with more to come later.
Despite his involvement in spurring their development via Operation Warp Speed, Trump largely downplayed vaccines during his final months in office, and both Trump and First Lady Melania Trump received the vaccine in private before Joe Biden took office as the new president in January 2021.
On February 27, 2021, the Janssen COVID-19 vaccine was granted emergency use authorization by the FDA for use. However, this vaccine has faced backlash from some government officials, believing it to be not as effective as Pfizer or Moderna. On March 5, the mayor of Detroit, Mike Duggan, rejected a shipment of the Janssen vaccine, saying, "Moderna and Pfizer are the best. And I am going to do everything I can to make sure the residents of the city of Detroit get the best." After backlash, Duggan declared he would no longer decline the vaccine.
On March 11, 2021, President Biden announced that he would direct all states to make vaccines available to all adults no later than May 1. On April 6 he said he would direct states to make all adults eligible for vaccination by April 19. This deadline was met after several states opened up vaccination to everyone 16 and above the same day.
On March 26, 2021 Ocugen submitted its master file for Covaxin a conventional inactivated vaccine to the FDA. In June the FDA suggested that the company go the longer route to gain full FDA approval instead of emergency use authorization.
As of May 2021, most experts thought the United States would be unable to achieve herd immunity, at least in the near term, given insufficient demand for the vaccines. On that note, many sources are saying the new variants last longer than 14 days, such as those found at home and abroad.
On July 6, Biden announced plans for more targeted outreach in order to reach under-vaccinated populations, such as shifting from larger mass clinics to having wider availability at community locations and events, and going "neighborhood by neighborhood, and oftentimes, door to door—literally knocking on doors—to get help to the remaining people protected from the virus"
On July 16, the FDA approved a request by Pfizer to give its vaccine a priority review designation, meaning that final approval of the vaccine would be pursued on an expedited timetable. While priority review processes usually take up to six months, it was expected that the Pfizer vaccine may be approved within weeks. On August 23, the FDA announced that it had formally approved the Pfizer vaccine for individuals 16 and over. It remains subject to EUA for individuals aged 12 to 15.
Incentives
Efforts have been made by private businesses to help encourage vaccination, including ridesharing services offering free transport to vaccine clinic sites, and promotional incentives and discounts being offered to those who present proof of a recent vaccination. Free items included beer, pizza, crawfish, donuts, and french fries. Some sports teams established partnerships to offer vaccine clinics at their venues on game days, with free tickets to games offered to those who use the clinics. Free cannabis was available in Washington State when getting a vaccine at a dispensary.
Massachusetts ran a free lottery for fully vaccinated people, and gave out $6.5 million to ten people in the form of cash and scholarships. Ohio ran a similar lottery, Maine gave away hunting licenses, and West Virginia gave away $100 savings bonds.
Vaccines on order
| Vaccine | Submitted (EUA) | Emergency use Authorization | Deployment | Submitted (Full) | Full Approval |
|---|---|---|---|---|---|
| Pfizer–BioNTech | |||||
| Moderna | |||||
| Janssen | |||||
| Covaxin | November 5, 2021 (ages 2-18) |
Pending | |||
| Novavax | Pending | ||||
| Astrazeneca | — | — | |||
| Sanofi–GSK |
Vaccine distribution
Listed in millions,
- As of February 16, 2022
- Pfizer–BioNTech
- Moderna
- Janssen
- Not Identified
Booster doses
On August 12, 2021, the FDA amended the EUA for the Moderna and Pfizer vaccines in order to allow a third, booster dose for patients who are immunocompromised or have received organ transplants. Director of the CDC Rochelle Walensky stated in a press briefing that "certain people who are immune compromised, such as people who have had an organ transplant and some cancer patients, may not have had an adequate immune response to just two doses of the ." In an interview with Today, Director of the National Institute of Allergy and Infectious Diseases (NIAID) Anthony Fauci stated that "inevitably, there will be a time when we'll have to give boosts . What we're doing literally on a weekly and monthly basis is following cohorts of patients to determine if, when and whom should get it. But right now at this moment, other than the immune compromised, we're not going to be giving boosters to people." The World Health Organization (WHO) discourages the use of booster shots for COVID-19 in developed countries, as they would divert supplies away from the unvaccinated.
On August 18, 2021, Walensky and acting Commissioner of the FDA Janet Woodcock released a joint statement indicating that, pending FDA approval, booster doses for the Moderna and Pfizer vaccines could become generally available by the week of September 20, for those who had received their second dose at least eight months prior. They stated that that protection against severe outcomes or death "could diminish in the months ahead, especially among those who are at higher risk or were vaccinated during the earlier phases of the vaccination rollout", such as health care workers and seniors.
On September 17, 2021, the FDA Vaccines and Related Biological Products Advisory Committee voted unanimously against recommending that booster doses of the Pfizer vaccine be distributed to the general public, citing the lack of evidence that a third dose is safe for younger populations. On September 23, Walensky officially approved the distribution of Pfizer booster doses to certain populations six months after their second dose, including those who are 65 and over, those with underlying conditions, and those who work in high-risk settings. On October 14, 2021, the committee recommended a half-dose booster for the Moderna vaccine among the same populations.
The next day, the committee also recommended that a second dose be generally distributed for the Janssen vaccine, at least two months after the first. An FDA analysis cited that a single dose of the vaccine had a consistently lower efficacy in comparison to the mRNA vaccines.
Timeline
| This section needs to be updated. Please help update this article to reflect recent events or newly available information. (February 2022) |


December 2020
On December 10, 2020, the FDA granted emergency use to the Pfizer vaccine. On December 14, 2020, the first vaccine doses were administered. Sandra Lindsay of Queens, New York City, was the first American to be administered an FDA-authorized COVID-19 vaccine. The Moderna vaccine was granted emergency use authorization three days later, on December 17.
On December 21, President-elect Joe Biden publicly received his first dose of the Pfizer vaccine during a media event at Christiana Hospital in Delaware. The event was intended as a means by the incoming Biden administration to boost public confidence in the vaccines. Biden stated that Trump "deserve some credit getting this off the ground with Operation Warp Speed", and told the press "It's going to take time, and in the meantime—I don't want to sound a sour note here—but I hope people listen to all the experts." Vice President-elect Kamala Harris received her first dose of the Moderna vaccine on December 29, also in public.
March 2021
On March 12, 2021, the United States surpassed 100 million doses administered.
Over the course of the month, seven states (Arkansas, Mississippi, Ohio, Connecticut, Arizona, Texas, and Georgia) made the vaccine available to all adults, following a period of selective vaccination for elderly and other vulnerable populations. California and Washington announced that all adults would be eligible for a vaccine starting April 15. Indiana announced that it would make vaccines available to university students and would specifically facilitate the transfer of vaccines to university-based vaccine clinics, including one such clinic at the University of Notre Dame. On Day 58 (March 19) of the Biden administration, it achieved its goal of administering 100 million doses within its first 100 days. On March 25, the administration set a new goal of administering 200 million doses in its first 100 days.
April 2021
On April 3, more than four million doses of the COVID-19 vaccine were reported administered in the past 24 hours, setting a new record and bringing the seven-day average to more than three million a day. As of April 11, more than 187 million vaccine doses have been administered. On April 13, the CDC and FDA issued a statement recommending a pause in the use of the Johnson & Johnson vaccine "out of an abundance of caution" after six women aged 18 through 48 developed a rare and severe type of blood clot called cerebral venous sinus thrombosis; on April 23, the recommended pause was lifted. By April 19, all U.S. states had made Americans aged 16 and older eligible for vaccination.
On April 22, the Biden administration announced that it had achieved its new goal of 200 million vaccine doses administered within its first 100 days. As of April 28, the CDC reported an average of 2.7 million daily vaccinations over the past week.
May 2021
On May 4, 2021, Biden announced a new target of having at least 70% of U.S. adults receive one vaccine dose by July 4.
On May 10, 2021, the FDA granted emergency use authorization for the Pfizer vaccine for use on adolescents aged 12–15, making the United States the second country in the world, after Canada, to do so. On May 12, Director of the CDC Rochelle Walensky approved the recommendation. On May 25, the Biden administration announced that 50% of adults had been fully vaccinated.
June 2021
By June 2021, the pace of vaccination had begun to decrease, amid increasing spread of the highly-infectious Delta variant across the country. On June 3, the White House announced additional private-sector partnerships in order to expand targeted outreach and vaccine availability, including an initiative to promote and offer vaccines through Black-owned barber shops throughout June, and a partnership with brewery Anheuser-Busch—who pledged in a promotional campaign to offer a "free beer" to all adults over 21 if the country met Biden's 70% goal.
The United States passed 600,000 Covid deaths on Tuesday, June 15, more than 200 times higher than the number of lives lost during the attacks of September 11, 2001. By late-June, one-quarter of COVID-19 cases in the U.S. were attributable to the Delta variant. Dr. Fauci warned that vaccination disparities could lead to "two Americas" where less vaccinated U.S. populations were at much higher risk of COVID-19.
July 2021
The U.S. missed Biden's 70% goal by approximately three million residents, or 3%. Only 18 states reached the 70% goal among their local population. Generally, the goal was met for adults 27 and over, but not for younger populations. By early-July 2021, the number of daily COVID-19 cases in the U.S. had declined by around 90% since the peak in January. However, cases had also begun to increase in parts of the country with below-average vaccination rates, and hesitancy towards the vaccine had become a partisan issue: a poll by The Washington Post and ABC News found that 86% of self-identified Democrats surveyed had received at least one dose, as opposed to 45% of self-identified Republicans, the 18 states that did reach 70% of first doses by July 4 were all won by Biden during the 2020 election, and a July 8 report by KFF found that the average vaccination rate in counties that voted for Biden was growing in comparison to those that voted for Trump.
Anti-vaccination rhetoric increased among conservatives, including Republican politicians and right-wing media outlets (such as Fox News and Newsmax) promoting misinformation and conspiracy theories (such as falsely claiming that the Biden administration would impose mandatory door-to-door vaccination—an exaggeration of Biden's recently announced plans for targeted outreach), Republican lawmakers attempting to inhibit vaccine outreach, and state laws to prohibit "discrimination" against the unvaccinated (which effectively ban vaccine mandates).
On July 15, Surgeon General Vivek Murthy warned that health-related misinformation was a public health threat. The next day, President Biden accused social networking service Facebook of "killing people" by enabling the spread of vaccine misinformation. On July 19, after criticism from Facebook over the comments, Biden stated that they were based on a report suggesting that approximately 12 social media users were responsible for at least 60% of vaccine misinformation, and clarified that "Facebook isn't killing people, these 12 people are out there giving misinformation. Anyone listening to it is getting hurt by it. It's killing people. It's bad information". He went on to explain that "My hope is that Facebook, instead of taking it personally that somehow I'm saying Facebook is killing people, that they would do something about the misinformation, the outrageous misinformation about the vaccine".
In late-July, amid increasing COVID-19 hospitalizations in a number of Republican-led states, some Republican politicians became more vocal in promoting vaccination, including Senate Minority Leader Mitch McConnell, Senator of Texas John Cornyn, and former Surgeon General Jerome Adams. Governor of Florida Ron DeSantis—who has been critical of Fauci and COVID-19 restrictions—stated in a press appearance that vaccines were "saving lives" and "reducing mortality" (citing the majority of hospitalizations being unvaccinated individuals), while criticizing mask mandates as displaying a lack of confidence in the efficacy of vaccines. McConnell used campaign funds to purchase radio advertisements in his home state of Kentucky to promote vaccination; the ad features McConnell discussing his childhood battle with Polio, and stating that the development of multiple safe and effective COVID-19 vaccines in such a short time was "nothing short of a modern medical miracle", in comparison to the "decades" it took to develop a Polio vaccine.
A number of conservative commentators also abruptly made statements in support of vaccination, including Fox News hosts Steve Doocy and Sean Hannity during broadcasts of Fox & Friends and Hannity respectively, Newsmax CEO and Trump confidant Christopher Ruddy (who praised Biden in an op-ed for "making a huge dent" in the pandemic by embracing and prioritizing the rollout of the "effective vaccine" inherited from the Trump presidency), and conservative commentator Ben Shapiro. However, Hannity subsequently walked back his remarks on his radio show and Hannity, arguing that he never urged his viewers to get the vaccine, and adding the caveat that they should get the vaccine "if it's right for you", after doing "all of your research" and speaking with medical professionals that they trust.
On July 26, 2021, a number of organizations announced that they would mandate the vaccination of their employees, including the American Medical Association and American Nurses Association, and frontline health care workers of the Department of Veterans Affairs. California and New York City announced that all government employees would be subject to weekly testing if they are not vaccinated.
On July 29, President Biden similarly announced that unvaccinated federal employees and on-site contractors will be subject to mandatory social distancing, masks, weekly or bi-weekly COVID-19 testing, and limitations on work travel. Biden cited Delta variant and a "pandemic of the unvaccinated" as justification for the measures. He also announced that the federal government planned to use funds from the American Rescue Plan to reimburse small and medium-sized businesses for paid time off for employee vaccine appointments (similar to paid time off provided for voting in elections), and called for states to offer $100 payments to the newly vaccinated using American Rescue Plan funding as an "extra incentive to boost vaccination rates, protect communities, and save lives." Biden stated that "I know that paying people to get vaccinated might sound unfair to folks that have gotten vaccinated already but here's the deal: if incentives help us beat this virus, I believe we should use them."
August 2021
On August 1, the CDC reported that there had been a 24% week-over-week increase in the number of first doses administered, especially in states with lower vaccination rates. As of August 2, at least 70% of U.S. adults had received at least one vaccine dose. On August 6, the CDC reported that half of the U.S population has been fully vaccinated. Several major cities announced or approved plans to restrict access to non-essential indoor locations such as cinemas, restaurants, and other entertainment venues, to those who are vaccinated.
The FDA gave full approval to the Pfizer-BioNTech vaccine on August 23 for patients 16 and older, prompting President Biden to encourage those who had been waiting for full approval to get vaccinated. Anthony Fauci projected that at least 20% of unvaccinated Americans "will now step forward and get vaccinated" due to the approval. With the approval, it was also announced that the Department of Defense was preparing guidance requiring the vaccination of military members for COVID-19, with press secretary John Kirby stating that this would "ensure the safety of our service members and promote the readiness of our force, not to mention the health and safety of the communities around the country in which we live."
October 2021
On October 20, the White House said it had enough Pfizer-BioNTech pediatric vaccine for every child in the country age 5–11 and that it expected federal health officials to approve the vaccine within weeks, upon which approval the White House planned to begin distributing the doses. Dr. Fauci said on October 24 that it seemed likely that the FDA might approve the vaccine for distribution in early November. On October 29, 2021, The U.S. Food and Drug Administration authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age. Vice President Harris received her booster shot of the Moderna vaccine on October 30.
Vaccination mandates
Main article: COVID-19 vaccination mandates in the United States These paragraphs are an excerpt from COVID-19 vaccination mandates in the United States. Over the course of the COVID-19 pandemic, COVID-19 vaccine mandates have been enacted by numerous states and municipalities in the United States, and also by private entities. In September 2021, President Joe Biden announced that the federal government would take steps to mandate COVID-19 vaccination for certain entities under the authority of the federal government or federal agencies. Most federal mandates thus imposed were either overturned through litigation, or withdrawn by the administration, although a mandate on health care workers in institutions receiving Medicare and Medicaid funds was upheld. All federal mandates were lifted when the national emergency was declared to have ended in May 2023. A small number of states have gone in the opposite direction, through executive orders or legislation designed to limit vaccination mandates.Vaccinations by state and territory
| This section may have misleading content. Please help clarify the content. (July 2021) |


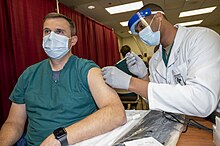
| State/Territory | Vaccinated | % of pop. |
|---|---|---|
| 2,489,954 | 58.1% | |
| 473,411 | 64.7% | |
| 4,858,243 | 66.7% | |
| 1,881,140 | 62.3% | |
| 32,416,845 | 82.0% | |
| 4,262,561 | 74.0% | |
| 3,128,940 | 87.8% | |
| 741,038 | 76.1% | |
| 15,887,600 | 74.0% | |
| 6,439,993 | 60.7% | |
| 1,219,631 | 86.1% | |
| 928,594 | 52.0% | |
| 9,069,775 | 71.6% | |
| 3,877,885 | 57.6% | |
| 2,035,520 | 64.5% | |
| 2,002,194 | 68.7% | |
| 2,773,832 | 62.1% | |
| 2,651,149 | 57.0% | |
| 1,145,891 | 85.2% | |
| 4,821,957 | 79.8% | |
| 6,188,074 | 89.8% | |
| 6,298,825 | 63.1% | |
| 4,005,557 | 71.0% | |
| 1,644,289 | 55.2% | |
| 3,805,955 | 62.0% | |
| 659,768 | 61.7% | |
| 1,276,272 | 66.0% | |
| 2,121,602 | 68.9% | |
| 1,314,238 | 95.0% | |
| 7,354,692 | 82.8% | |
| 1,681,126 | 80.2% | |
| 16,126,203 | 82.9% | |
| 7,906,885 | 75.4% | |
| 471,496 | 61.9% | |
| 7,024,797 | 60.1% | |
| 2,589,735 | 65.4% | |
| 3,106,530 | 73.7% | |
| 9,898,704 | 77.3% | |
| 929,695 | 87.8% | |
| 3,208,049 | 62.3% | |
| 621,704 | 70.3% | |
| 3,988,659 | 58.4% | |
| 19,222,170 | 66.3% | |
| 2,144,450 | 66.9% | |
| 553,280 | 88.7% | |
| 6,688,229 | 78.4% | |
| 5,726,746 | 75.2% | |
| 1,103,589 | 61.6% | |
| 3,947,193 | 67.8% | |
| 321,280 | 55.5% | |
| 50 states | 235,035,945 | 70.5% |
| 40,116 | 84.6% | |
| 615,813 | 87.3% | |
| 140,416 | 83.3% | |
| 43,069 | 83.1% | |
| 2,821,540 | 88.3% | |
| 62,918 | 59.2% | |
| U.S. territories | 3,723,872 | 81.0% |
| 238,759,817 | 75.7% | |
| 26,005 | 33.5% | |
| 48,364 | 47.3% | |
| 18,739 | 87.0% | |
| Compact of Free Association | 93,108 | 55.9% |
| USA + CoFA | 238,852,925 | 65.8% |
Vaccinations in the U.S. military
On August 9, 2021, all servicemembers received a memo explaining that, under a plan endorsed by President Biden and by military leadership, COVID-19 vaccination would become mandatory within about a month. About a third of active U.S. military service members had already been vaccinated as of late April; and about two-thirds (73% of all service members) had already been vaccinated by the time the memo was sent.
The U.S. Navy had been the fastest to begin vaccination in early 2021. As of April 22, 2021, considering active military personnel who had received at least one dose, the U.S. Navy had the highest percentage at 51%, the Marines at 36%, the Air Force/Space Force at 34%, and the Army at 27%. By late May, at least 58% of active military personnel had received at least one dose of the COVID-19 vaccine.
As of April 9, 2021, 39% of U.S. Marines to whom the military offered the vaccine had refused it. The highest rate of refusal was at Camp Lejeune in North Carolina, where 57% of Marines had refused the vaccine.
On August 23, 2021, the Pfizer-BioTech vaccine got its full FDA approval, prompting vaccinations to be required for all active duty, reserve, and National Guard troops starting August 25. By the time the vaccination requirement order was sent out, only 68% of active-duty troops were fully vaccinated.
On September 1, 2021, the U.S. Navy mandated vaccination of all active-duty Navy sailors and U.S. Marines by November 28 and of reservists by December 28. On September 3, 2021, the U.S. Air Force mandated vaccination of all active-duty airmen and U.S. Space Force Guardians by November 2, while Air Force reservists had until December 2. On September 14, 2021, the U.S. Army mandated vaccination of all active duty soldiers by December 15 and of reservists by June 30, 2022.
Vaccine certificates
Main article: COVID-19 vaccine cardThe U.S. does not issue a federally mandated COVID-19 certificate nor a digital proof that would allow venues to check the vaccine status of participants consistently. CDC vaccine cards that contain personal information including patient numbers and place of vaccinations are accepted in those European countries where American military personnel are stationed. However, those cards are not immediately transferrable to EU Digital COVID Certificates since CDC cards are not digitally validated and do not contain a counterfeit-proof or unique identifier. Asian countries have similar health certificate requirements for entering their territories. The lack of a federal system for checking COVID-19 status has put the United States in a disadvantage compared to nations in Europe and Asia.
Public opinion
| This section needs to be updated. Please help update this article to reflect recent events or newly available information. (February 2022) |
The Kaiser Family Foundation conducts ongoing polling and analysis in a project called the KFF COVID-19 Vaccine Monitor. It considers demographics including age, race, political party, education, and insurance status when examining vaccine hesitancy. A KFF study in December 2020 showed that 27% of the general public, and 29% of health careworkers, were hesitant to be vaccinated.
In December 2020 a Gallup poll found that 63% of Americans were willing to receive the COVID-19 vaccine.
Safety concerns about the currently available vaccines are a primary factor in vaccine hesitancy and refusal. Most unvaccinated adults are not confident in the safety of the available vaccines and while 63% of parents are confident the vaccines are safe for adults only about half of those who are confident in safety for adults are confident they are safe for ages 12-17 and its further reduced for even younger age groups. The need for more research for the vaccines is the most stated reason given by parents for not wanting to have their child vaccinated.
Political leanings are reflected in vaccine hesitancy. Early in the pandemic, before vaccines were available, a poll conducted May 20–21, 2020, found that 44% of Republicans and 19% of Democrats believed a debunked conspiracy theory that Bill Gates was plotting to use a COVID-19 vaccine to inject microchips into the population. Months after vaccination began, a Monmouth poll conducted April 8–12, 2021, found that two-thirds of Democratic voters had already been vaccinated but only one-third of Republican voters had done so. A Quinnipiac poll conducted on the same dates found that 45% of Republicans said they did not plan to be vaccinated. The New York Times wrote that the vaccination program was "hitting what appears to be a soft ceiling" as it moved to dealing with the demographic groups where vaccine hesitancy was stronger.
In January 2021, approximately one third of the population was concerned the vaccine might be made mandatory.
A Frank Luntz poll in mid-April 2021 found a rise in vaccine confidence from the previous month, despite the pause of the Johnson and Johnson vaccine.
A later poll by The Washington Post and ABC News found that 86% of self-identified Democrats surveyed had received at least one dose, as opposed to 45% of self-identified Republicans.
In July 2021, The New York Times noted that there were two groups of Americans who remained hesitant to get the vaccine, the first group being "a mix of people but tend to be disproportionately white, rural, evangelical Christian and politically conservative" and the second group being "a broad range of people, but tend to be a more diverse and urban group, including many younger people, Black and Latino Americans, and Democrats."
See also
- Deployment of COVID-19 vaccines
- COVID-19 pandemic in the United States
- COVID-19 vaccine
- Ohio Vax-A-Million
References
- COVID-19 Vaccinations in the United States. By Centers for Disease Control and Prevention. Percent of people receiving at least one COVID-19 vaccine dose by state or territory for the total population. Hover or click on the states or territories for more info. For data table scroll down to "Data Table for COVID-19 Vaccinations in the United States".
- "Interior Applauds Inclusion of Insular Areas through Operation Warp Speed to Receive COVID-19 Vaccines" (Press release). United States Department of the Interior (DOI). December 12, 2020. Retrieved January 13, 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Dorman B (January 6, 2021). "Asia Minute: Palau Administers Vaccines to Keep Country Free of COVID". Hawaii Public Radio. Retrieved January 13, 2021.
- ^ "COVID Data Tracker". Centers for Disease Control and Prevention. March 28, 2020. Retrieved April 23, 2021.
- Thomas, Katie; Weiland, Noah; LaFraniere, Sharon (December 10, 2020). "F.D.A. Advisory Panel Gives Green Light to Pfizer Vaccine". The New York Times.
- Lovelace, Berkeley Jr. (December 19, 2020). "FDA approves second Covid vaccine for emergency use as it clears Moderna's for U.S. distribution". CNBC.
- Machemer, Theresa. "FDA Approves Johnson & Johnson Vaccine, Another Valuable Tool Against Covid-19". Smithsonian Magazine.
- ^ Anthes, Emily; Ngo, Madeleine; Sullivan, Eileen (April 19, 2021). "Adults in all U.S. states are now eligible for vaccination, hitting Biden's target. Half have had at least one dose". The New York Times. ISSN 0362-4331. Retrieved May 22, 2021.
- Johnson, Carolyn Y. (May 10, 2021). "FDA authorizes Pfizer coronavirus vaccine for adolescents 12 to 15 years old". The Washington Post. Retrieved May 10, 2021 – via MSN.
- ^ Lovelace, Berkeley Jr. (August 23, 2021). "FDA grants full approval to Pfizer-BioNTech's Covid shot, clearing path to more vaccine mandates". CNBC. Retrieved August 23, 2021.
- Betsy Klein, Veronica Stracqualursi and Kate Sullivan. "Biden unveils Covid-19 plan based on 'science not politics' as he signs new initiatives". CNN. Retrieved March 12, 2021.
- "COVID-19: US president Joe Biden signs 10 executive orders to curb spread of coronavirus". Sky News. Retrieved March 12, 2021.
- "100 Million Shots In 100 Days: Is Biden's COVID-19 Vaccination Goal Achievable?". NPR. Retrieved March 12, 2021.
- "Biden Eyes New Goal After US Clears 100M Shots Since Jan. 20". HuffPost. March 20, 2021.
- Dan Mangan; Berkeley Lovelace Jr. (March 25, 2021). "Biden sets new Covid vaccine goal of 200 million shots within his first 100 days: 'I believe we can do it'". CNBC. Retrieved March 27, 2021.
- Pettypiece, Shannon; Shabad, Rebecca (April 21, 2021). "'We did it': Biden celebrates U.S. hitting milestone of 200 million doses in his first 100 days". NBCNews.com. Retrieved April 22, 2021.
- "Biden announces sweeping vaccine mandates affecting millions of workers". NBC News. September 13, 2021. Archived from the original on September 13, 2021. Retrieved January 27, 2022.
- "Federal Register :: COVID-19 Vaccination and Testing; Emergency Temporary Standard". January 27, 2022. Archived from the original on January 27, 2022. Retrieved January 27, 2022.
- "21A244 National Federation of Independent Business v. OSHA (01/13/2022)" (PDF). Archived from the original (PDF) on January 13, 2022. Retrieved January 27, 2022.
- Schneider, Eric C.; Shah, Arnav; Sah, Pratha; Moghadas, Seyed M.; Vilches, Thomas; Galvani, Alison (December 14, 2021). "The U.S. COVID-19 Vaccination Program at One Year: How Many Deaths and Hospitalizations Were Averted?". The Commonwealth Fund. Retrieved February 8, 2022.
- U.S. COVID-19 vaccine tracker: See your state’s progress. From the Mayo Clinic. Hover on the states on the map at the source page for exact numbers for the fully vaccinated. Click on the "data sources" link in the first paragraph to see that the data is from the Centers for Disease Control and Prevention.
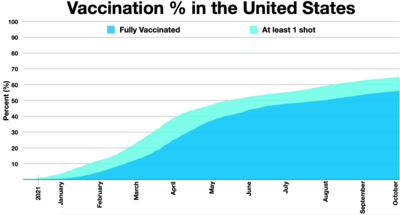
- Timeline of daily COVID-19 vaccine doses administered in the US. Click on the chart tab. Then click the download tab, and then the SVG link to get the SVG file. The table tab has a table of the data by date. The sources tab says the data is from the COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. The graph on the chart tab at the source is interactive and provides more detail. For example, run your cursor over the graph to see the date and the number vaccinated that day. The actual number may be higher or lower since a rolling 7-day average is used.
- Bertsimas, Dimitris; Digalakis, Vassilis Jr.; Jacquillat, Alexander; Li, Michael Lingzhi; Previero, Alessandro (2021). "Where to locate COVID-19 mass vaccination facilities?". Naval Research Logistics. 69 (2): 179–200. arXiv:2102.07309. doi:10.1002/nav.22007. ISSN 1520-6750. PMC 8441649.
- Elliott, Christopher (April 2, 2021). "Vaccine passports: How to prove you've gotten your COVID-19 shots for travel and avoid scams". USA Today. Retrieved July 16, 2021.
- Otterman, Sharon (June 1, 2021). "Will the Excelsior Pass, New York's Vaccine Passport, Catch On?". The New York Times. Retrieved July 24, 2021.
- Lee, Bruce Y. (June 20, 2021). "California Now Has A Digital Covid-19 Vaccine Record System". Forbes. Retrieved July 24, 2021.
- "DHS Statement on Equal Access to COVID-19 Vaccines and Vaccine Distribution Sites". Department of Homeland Security. February 1, 2021. Retrieved April 29, 2021.
- "Licensed Drivers | Bureau of Transportation Statistics". www.bts.gov. Retrieved April 29, 2021.
- "Verdades y mentiras sobre la supuesta vacuna para turistas en EE. UU". El Tiempo (in Spanish). April 27, 2021. Retrieved April 29, 2021.
- "Coronavirus: las medidas en EE. UU. contra el 'turismo de vacunas'". El Tiempo (in Spanish). February 2, 2021. Retrieved April 29, 2021.
- "Estados Unidos no les canceló visas a latinos que viajaron a vacunarse". El Tiempo (in Spanish). April 16, 2021. Retrieved April 29, 2021.
- "Así vacunan a extranjeros en las principales ciudades de EE. UU". El Tiempo (in Spanish). May 13, 2021. Retrieved May 17, 2021.
- Aquino, Marco; Esposito, Anthony; Garrison, Cassandra (May 11, 2021). "'Want the COVID-19 vaccine? Have a U.S. visa?' Latinos travel north for the shot". Reuters. Retrieved May 17, 2021.
- Honan, Katie (May 6, 2021). "New York City to Offer Covid-19 Vaccines to Tourists". The Wall Street Journal. ISSN 0099-9660. Retrieved May 17, 2021.
- Romo, Vanessa (June 3, 2021). "Alaska Lures Tourists With Its Stunning Landscapes — And Free COVID-19 Vaccines". WSHU Public Radio. Retrieved June 29, 2021.
- "COVID-19 (coronavirus) vaccine: Get the facts". Mayo Clinic. April 22, 2020.
- ^ Gottlieb, Scott (April 26, 2020). "America Needs to Win the Coronavirus Vaccine Race". The Wall Street Journal.
- Yamamoto, Vicky; Bolanos, Joe F.; Fiallos, John; Strand, Susanne E.; Morris, Kevin; Shahrokhinia, Sanam; Cushing, Tim R.; Hopp, Lawrence; Tiwari, Ambooj; Hariri, Robert; Sokolov, Rick (January 1, 2020). "COVID-19: Review of a 21st Century Pandemic from Etiology to Neuro-psychiatric Implications". Journal of Alzheimer's Disease. 77 (2): 459–504. doi:10.3233/JAD-200831. ISSN 1387-2877. PMC 7592693. PMID 32925078.
- Sanger, David E.; Kirkpatrick, David D.; Wee, Sui-Lee; Bennhold, Katrin (March 19, 2020). "Search for Coronavirus Vaccine Becomes a Global Competition". The New York Times. Retrieved August 15, 2020.
- "Guide to the Cares Act". United States Senate Committee on Small Business and Entrepreneurship. Retrieved August 15, 2020.
- Gutierrez, Gabe; Seidman, Joel (March 21, 2020). "Walter Reed researchers join global search for coronavirus vaccine". NBC News. Retrieved December 10, 2020.
- De Paoli, Lucca (April 26, 2020). "Bill Gates's Coronavirus Vaccine Could Be Ready in 12 Months". Bloomberg News. Retrieved December 7, 2020.
- "Johnson & Johnson reaches deal with U.S. for 100 million doses of coronavirus vaccine at more than $1 billion". CNBC. August 5, 2020. Retrieved August 5, 2020.
- LaFraniere, Sharon; Weiland, Noah (October 5, 2020). "White House Blocks New Coronavirus Vaccine Guidelines". The New York Times. Retrieved October 6, 2020.
- Armstrong, Drew; LaVito, Angelica (October 6, 2020). "FDA Sets Goals That May Put Vaccine Out of Reach Before Election". Bloomberg News.
- Zimmer, Carl (October 6, 2020). "The F.D.A. has released stricter guidelines for vaccine developers after a holdup at White House". The New York Times. Retrieved October 6, 2020.
- U.S. Department of Health and Human Services: Food and Drug Administration: Center for Biologics Evaluation and Research (June 2020). "Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry". FDA. Retrieved October 6, 2020.
- Corum, Jonathan; Wee, Sui-Lee; Zimmer, Carl (October 2020). "Coronavirus Vaccine Tracker". The New York Times. Retrieved October 6, 2020.
- Office of the Commissioner (May 13, 2021). "Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic". FDA. Retrieved May 21, 2021.
- "Pfizer and BioNTech to Submit Emergency Use Authorization Request Today to the U.S. FDA for COVID-19 Vaccine". Pfizer (Press release). November 20, 2020. Retrieved November 20, 2020.
- Park A (November 20, 2020). "Exclusive: Pfizer CEO Discusses Submitting the First COVID-19 Vaccine Clearance Request to the FDA". Time. Retrieved November 20, 2020.
- "Coronavirus (COVID-19) Update: FDA Announces Advisory Committee Meeting to Discuss COVID-19 Vaccine Candidate". U.S. Food and Drug Administration (FDA) (Press release). Retrieved November 20, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- "Vaccines and Related Biological Products Advisory Committee December 10, 2020 Meeting Announcement". U.S. Food and Drug Administration (FDA). Retrieved November 30, 2020.
- Hirsch, Lauren (June 1, 2021). "E.E.O.C. Says Companies Can Mandate Vaccines, but Few Push Ahead". The New York Times. Retrieved July 10, 2021.
- "FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine" (Press release). U.S. Food and Drug Administration (FDA). December 11, 2020. Retrieved December 11, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. (December 2020). "The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Pfizer–BioNTech COVID-19 Vaccine – United States, December 2020" (PDF). MMWR Morb Mortal Wkly Rep. 69 (50): 1922–24. doi:10.15585/mmwr.mm6950e2. PMC 7745957. PMID 33332292.
- Thomas, Katie; LaFraniere, Sharon; Weiland, Noah; Goodnough, Abby; Haberman, Maggie (December 12, 2020). "F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away". The New York Times. ISSN 0362-4331. Retrieved December 12, 2020.
- Maxouris, Christina (December 12, 2020). "FDA's Covid-19 vaccine authorization is a 'monumental moment', expert says. It came on the deadliest day of the pandemic". CNN. Retrieved December 12, 2020.
- Stanley-Becker I, Abutaleb Y, Sun LH, Dawsey J (December 17, 2020). "States report confusion as government reduces vaccine shipments, while Pfizer says it has 'millions' of unclaimed doses". The Washington Post. Retrieved December 19, 2020.
- ""We are not perfect": General apologizes for "miscommunication" over vaccine shipments". Axios. December 19, 2020. Retrieved December 19, 2020.
- "FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine". U.S. Food and Drug Administration (FDA) (Press release). Retrieved December 18, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. (December 2020). "The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine – United States, December 2020" (PDF). MMWR. Morbidity and Mortality Weekly Report. 69 (5152): 1653–1656. doi:10.15585/mmwr.mm695152e1. PMID 33382675. S2CID 229945697.
- "Moderna Applies for Emergency F.D.A. Approval for Its Coronavirus Vaccine". The New York Times. November 30, 2020. Retrieved November 30, 2020.
- "Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization". Moderna, Inc. (Press release). November 30, 2020. Retrieved November 30, 2020.
- Lovelace, Berkeley Jr. (December 19, 2020). "FDA approves second Covid vaccine for emergency use as it clears Moderna's for U.S. distribution". CNBC. Retrieved December 19, 2020.
- "Trump Administration purchases additional 100 million doses of COVID-19 investigational vaccine from Moderna". U.S. Health and Human Services (HHS). December 11, 2020. Retrieved December 18, 2020.
- ^ Cancryn, Adam. "GOP sees widening rift over promoting Covid shots". POLITICO. Retrieved July 24, 2021.
- ^ Graham, David A. (July 20, 2021). "Suddenly, Conservatives Care About Vaccines". The Atlantic. Retrieved July 24, 2021.
- Haberman, Maggie (March 1, 2021). "Trump and his wife received coronavirus vaccine before leaving the White House". The New York Times. ISSN 0362-4331. Retrieved March 7, 2021.
- "Detroit mayor declines Johnson & Johnson allotment, saying the other vaccines are better". KTVZ. March 5, 2021. Retrieved March 5, 2021.
- Frank, Annalise (March 5, 2021). "Detroit Mayor Duggan walks back comments, now says he'll welcome Johnson & Johnson vaccine". Crain's Detroit Business. Retrieved March 5, 2021.
- Sullivan, Sean. "Biden directs states to make all adults eligible for vaccine by May 1". The Washington Post. ISSN 0190-8286. Retrieved March 12, 2021.
- Collins, Kaitlan; Sullivan, Kate; Vazquez, Maegan. "Biden moves deadline for all US adults to be eligible for Covid vaccine to April 19". CNN.
- Allassan, Fadel. "All U.S. adults now eligible for COVID-19 vaccine, meeting Biden's April 19 deadline". Axios. Retrieved April 20, 2021.
- ^ "Ocugen On Track to Submit Emergency Use Authorization Application to U.S. FDA for its COVID-19 Vaccine Candidate, COVAXIN™ | Ocugen, Inc". ir.ocugen.com.
- Mandavilli, Apoorva (May 3, 2021). "Reaching 'Herd Immunity' Is Unlikely in the U.S., Experts Now Believe". The New York Times. ISSN 0362-4331. Retrieved May 13, 2021.
- "Healthcare Workers". Centers for Disease Control and Prevention. February 11, 2020. Retrieved May 13, 2021.
- ^ Holmes Lybrand and Tara Subramaniam. "Fact-checking Republicans' false narratives around Biden's door-to-door vaccine efforts". CNN. Retrieved July 19, 2021.
- "Vaccines door-to-door: Immunization push goes granular as delta variant looms". Washington Post. July 6, 2021. Retrieved July 19, 2021.
- Coleman, Justine (July 9, 2021). "Freedom Caucus chair attacks Biden actions on vaccines". TheHill. Retrieved July 19, 2021.
- Fritts, Rachel (July 21, 2021). "When will COVID-19 vaccines be fully approved—and does it matter whether they are?". Science | AAAS. Retrieved August 16, 2021.
- Ayala, Christine (August 11, 2021). "How 'waiting for full FDA approval' was weaponized by vaccine opponents". TheHill. Retrieved August 16, 2021.
- LaFraniere, Sharon; Weiland, Noah (August 3, 2021). "F.D.A. Aims to Give Final Approval to Pfizer Vaccine by Early Next Month". The New York Times. ISSN 0362-4331. Retrieved August 16, 2021.
- "FDA Approves First COVID-19 Vaccine" (Press release). U.S. Food and Drug Administration (FDA). August 23, 2021. Archived from the original on August 23, 2021. Retrieved August 23, 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Wiener-Bronner, Danielle. "Krispy Kreme has given away over 1.5 million doughnuts to vaccinated people". CNN. Retrieved July 2, 2021.
- Landers, Mary. "The perks of getting the COVID vaccine go beyond health". Savannah Morning News. Retrieved July 19, 2021.
- ^ "A 'Shot' At $1 Million? Local Governments Offering Incentives For Vaccines". NPR.
- "Mariners offer vaccinations during games". Major League Baseball. Retrieved May 10, 2021.
- McKinley, Edward (May 5, 2021). "Mets, Yankees, outdoor stadiums can go to full capacity for vaccinated fans". Times Union. Retrieved May 8, 2021.
- "Washington announces cannabis giveaway in state-approved 'joint for jabs' vaccine campaign". www.msn.com.
- Klein • •, Asher. "Who Are the Final VaxMillions Winners? They've Just Been Revealed".
- "Ocugen, Inc. Announces Submission of Emergency Use Authorization Request to the US FDA for Investigational COVID-19 Vaccine COVAXIN™ (BBV152) for Children Ages 2-18 Years | Ocugen, Inc". ir.ocugen.com.
- "National Weekly Pfizer Allocations". data.cdc.gov. Retrieved March 5, 2021.
- "National Weekly Moderna Allocation". data.cdc.gov. Retrieved March 5, 2021.
- Howard, Jacqueline. "FDA authorizes additional Covid-19 vaccine doses for certain immunocompromised people". CNN. Retrieved August 13, 2021.
- "Why A Push For Boosters Could Make The Pandemic Even Worse". NPR. Retrieved August 18, 2021.
- "US health officials call for booster shots against COVID-19". apnews.com. August 18, 2021.
- "FDA advisory group rejects Covid boosters for most, limits to high-risk groups". NBC News. Retrieved October 18, 2021.
- Robert Towey; Berkeley Lovelace Jr. (September 23, 2021). "The leader of CDC just made a rare call to allow Covid booster shots for more people". CNBC. Retrieved October 18, 2021.
- "FDA panel backs half-dose Moderna booster for older and at-risk US adults". The Guardian. October 14, 2021. Retrieved October 18, 2021.
- Robert Towey; Berkeley Lovelace Jr. (October 15, 2021). "FDA panel unanimously recommends J&J Covid booster shots to adults who already got the first dose". CNBC. Retrieved October 18, 2021.
- Fox, Maggie. "FDA advisers recommend J&J Covid-19 vaccine boosters, talk mix and match boosters". CNN. Retrieved October 18, 2021.
- The Straits Times staff (December 30, 2020). "Kamala Harris vaccinated on camera, urges public to trust process". The Straits Times. Retrieved February 3, 2021.
- Bekiempis, Victoria (December 21, 2020). "'I'm ready': Joe Biden receives coronavirus vaccine live on TV". The Guardian. Retrieved February 3, 2021.
- Aratani, Lauren (December 18, 2020). "Mike Pence receives Covid-19 vaccine on live TV: 'I didn't feel a thing'". The Guardian. Retrieved February 3, 2021.
- Pereira, Ivan (December 14, 2020). "US administers 1st doses of Pfizer coronavirus vaccine". ABC News. Retrieved March 6, 2021.
- "Emergency Use Authorization for Vaccines to Prevent COVID-19. Guidance for Industry" (PDF). www.fda.gov. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. May 25, 2021. Retrieved May 20, 2021.
- Mir, Alice; Ollstein, A. "Biden to receive coronavirus vaccine in public on Monday". POLITICO. Retrieved July 24, 2021.
- Higgins, Tucker (December 21, 2020). "Joe Biden receives Covid vaccine on live television, encourages Americans to get inoculated". CNBC. Retrieved July 24, 2021.
- Holveck, Brandon. "Biden receives COVID-19 vaccine at ChristianaCare Monday afternoon". The News Journal. Retrieved July 24, 2021.
- "U.S. surpasses 100 million COVID-19 vaccines administered". www.pharmacist.com. Retrieved March 18, 2021.
- "Dunleavy Announces COVID-19 Vaccine Available to All Alaskans". Archived from the original on March 23, 2021.
- "UPDATE: Mississippi State extends vaccine distribution criteria in accordance with Gov. Reeves' announcement". March 15, 2021. Archived from the original on March 16, 2021.
- "Everyone 16+ in Ohio can now schedule for COVID-19 vaccine if slots are available, Gov. Mike DeWine announces". March 22, 2021. Archived from the original on March 24, 2021.
- "Texas COVID-19 vaccine available for all adults starting March 29". March 23, 2021. Archived from the original on March 24, 2021.
- "All Georgians over age of 16 eligible to receive COVID-19 vaccines starting Thursday". WGAU. March 23, 2021.
- "All California adults can get COVID-19 vaccine next month". Los Angeles Times. March 25, 2021. Archived from the original on March 28, 2021.
- "ISDH to give Notre Dame enough COVID-19 shots to fully vaccinate all students". WTHR. March 30, 2021.
- Madeline Holcombe and Ray Sanchez. "US hits 4 million Covid-19 vaccine doses in a day for a new record". CNN. Retrieved April 4, 2021.
- "COVID-19 Vaccinations in the United States". CDC. March 28, 2020.
- Neuman, Scott (April 13, 2021). "U.S. Recommends Pausing Use of Johnson & Johnson Vaccine Over Blood Clot Concerns". NPR.
- Weiland, Noah; LaFraniere, Sharon; Zimmer, Carl (April 13, 2021). "U.S. Calls for Pause on Johnson & Johnson Vaccine After Clotting Cases". The New York Times. ISSN 0362-4331. Archived from the original on December 28, 2021. Retrieved April 13, 2021.
- "FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review". FDA. April 26, 2021. Retrieved May 22, 2021.
- "COVID Data Tracker". Centers for Disease Control and Prevention. March 28, 2020. Retrieved April 29, 2021.
- Berkeley Lovelace Jr; Kevin Breuninger (May 4, 2021). "Biden's new Covid vaccination goal is for 70% of adults to have at least one shot by July 4". CNBC. Retrieved July 19, 2021.
- Lovelace, Berkeley Jr (May 10, 2021). "FDA permits use of the Pfizer-BioNTech Covid vaccine in kids ages 12 to 15". CNBC. Retrieved July 19, 2021.
- Lovelace, Berkeley Jr. (May 12, 2021). "CDC OKs Pfizer's Covid vaccine for use in adolescents, clearing way for shots to begin Thursday". CNBC. Retrieved July 19, 2021.
- ^ Analysis by Stephen Collinson. "Analysis: 'Get a shot and have a beer': Biden's new strategy woos vaccine skeptics". CNN. Retrieved July 19, 2021.
- Jacqueline Howard. "Health officials turn to barber shops, hair salons as possible Covid-19 vaccination sites". CNN. Retrieved July 19, 2021.
- Elamroussi, Aya (June 30, 2021). "Fauci warns there may soon be 'two Americas' as divide widens between vaccinated and unvaccinated areas". CNN. Retrieved June 30, 2021.
- ^ "Biden didn't "fall short" of July 4 vaccination goal — he was sabotaged by Republican trolls". Salon. July 6, 2021. Retrieved July 19, 2021.
- ^ Collins, Michael; Garrison, Joey (June 22, 2021). "Biden administration says it won't make 70% COVID-19 vaccine goal. What we know". USA Today. Retrieved June 24, 2021.
- "The Red/Blue Divide in COVID-19 Vaccination Rates is Growing". KFF. July 8, 2021. Retrieved July 20, 2021.
- Jones, Sarah (July 12, 2021). "The Anti-Vaxx Party Is Taking Shape". Intelligencer. Retrieved July 19, 2021.
- Hsu, Tiffany (July 11, 2021). "Despite Outbreaks Among Unvaccinated, Fox News Hosts Smear Shots". The New York Times. ISSN 0362-4331. Retrieved July 19, 2021.
- ^ Medina, Daniella. "'Make polio great again'?: Americans outraged as Tennessee abandons vaccine outreach to teens". The Tennessean. Retrieved July 19, 2021.
- "Misinformation, vaccine accessibility hurdles result in hundreds of COVID-19 deaths each week". Salon. June 30, 2021. Retrieved July 19, 2021.
- Kang, Cecilia (July 18, 2021). "Facebook Tells Biden: 'Facebook Is Not the Reason' Vaccination Goal Was Missed". The New York Times. ISSN 0362-4331. Retrieved July 19, 2021.
- Owens, Caitlin. "Republicans push to ban "discrimination" against unvaccinated people". Axios. Retrieved July 19, 2021.
- ^ Breuninger, Kevin (July 19, 2021). "'Facebook isn't killing people' — Biden walks back attack over vaccine lies". CNBC. Retrieved July 19, 2021.
- Kaitlan Collins and Donie O'Sullivan. "Surgeon general issues warning over vaccine misinformation as White House turns up the heat on Facebook". CNN. Retrieved July 19, 2021.
- Rodriguez, Salvador (July 16, 2021). "Biden on Facebook: 'They're killing people' with vaccine misinformation". CNBC. Retrieved July 19, 2021.
- ^ Zaslav, Ali. "McConnell cites experience with polio in ad pushing Kentuckians to get vaccinated". CNN. Retrieved July 29, 2021.
- Oshin, Olafimihan (July 21, 2021). "DeSantis urges public to get vaccinated: These shots are 'saving lives'". TheHill. Retrieved July 24, 2021.
- Wilkie, Christina (July 28, 2021). "McConnell will use campaign funds to promote Covid vaccinations in Kentucky". CNBC. Retrieved July 29, 2021.
- Mastrangelo, Dominick (July 20, 2021). "Newsmax CEO: Biden 'should be applauded' for vaccine efforts". TheHill. Retrieved July 24, 2021.
- Whitehouse, John. "Sean Hannity distances himself from any kind of responsible COVID-19 vaccine position". Media Matters for America. Retrieved July 24, 2021.
- Baragona, Justin (July 23, 2021). "Hannity Makes Vax Stance 'Very Clear': 'I Never Told Anyone to Get a Vaccine!'". The Daily Beast. Retrieved August 19, 2021.
- Swanson, Ian (July 27, 2021). "Vaccine mandate calls fueled by COVID-19's latest spike". TheHill. Retrieved July 27, 2021.
- Mole, Beth (July 26, 2021). "COVID surge in unvaccinated is pushing US to more mandates, masks, mitigation". Ars Technica. Retrieved July 27, 2021.
- Rowland, Geoffrey (July 29, 2021). "Biden tells federal workers: Get vaccinated or submit to testing". TheHill. Retrieved July 29, 2021.
- "Covid-19: Biden tells states to offer $100 vaccine incentive as cases rise". BBC News. July 29, 2021. Retrieved July 29, 2021.
- Lejeune, Tristan (July 29, 2021). "Biden calls on states to offer $100 vaccine incentives". TheHill. Retrieved July 29, 2021.
- "FACT SHEET: President Biden to Announce New Actions to Get More Americans Vaccinated and Slow the Spread of the Delta Variant". The White House. July 29, 2021. Retrieved July 29, 2021.
- Nate Rattner; Berkeley Lovelace Jr (August 2, 2021). "U.S. reaches 70% Covid vaccine milestone for adults about a month behind Biden's goal". CNBC. Retrieved August 3, 2021.
- Chamberlain, Samuel (August 7, 2021). "Half of US population is fully vaccinated against COVID-19, CDC says". New York Post. Retrieved August 7, 2021.
- Tapp, Tom (August 11, 2021). "Los Angeles City Council Votes 13-0 To Create Vaccination Requirement For Indoor Public Spaces Such As Restaurants, Movie Theaters, Concert Venues". Deadline. Retrieved August 13, 2021.
- Thadani, Trisha (August 12, 2021). "San Francisco to become first major U.S. city to mandate full vaccination for many indoor activities". San Francisco Chronicle. Retrieved August 13, 2021.
- Benveniste, Alexis. "New York City will require vaccines for entry to restaurants and gyms". CNN. Retrieved August 13, 2021.
- "Pfizer-BioNTech's COVID Vaccine Gets Full Approval From The FDA". NPR. Retrieved August 24, 2021.
- Shivaram, Deepa (August 24, 2021). "Why Pfizer's FDA Approval Matters And What It Means For Vaccine Mandates". NPR. Retrieved August 24, 2021.
- Swanson, Ian (August 23, 2021). "Pentagon to mandate COVID-19 vaccine for military". TheHill. Retrieved August 24, 2021.
- Wang, Amy B; Sun, Lena H. (October 20, 2021). "White House unveils plans to roll out coronavirus vaccines for children ages 5 to 11". Washington Post. Retrieved October 20, 2021.
- Visser, Nick (October 25, 2021). "Fauci Suggests Timetable For 5- To 11-Year-Olds To Get Pfizer Shot". HuffPost. Retrieved October 25, 2021.
- "FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age". FDA. October 29, 2021. Retrieved November 8, 2021.
- "COVID Data Tracker". March 28, 2020.
- Baldor, Lolita (August 10, 2021). "Pentagon Issues COVID-19 Vaccine Mandate For All U.S. Military Members". HuffPost. Retrieved August 10, 2021.
- ^ Kime, Patricia (April 22, 2021). "Navy Pulls Way Ahead of the Other Military Services in Race to 100% Vaccination". Military.com.
- "Military's COVID Vaccination Rate Lags Behind Pentagon's Hopes". July 16, 2021.
- "DOD Seeing Encouraging COVID-19 Vaccination Rates, Health Leaders Say". U.S. DEPARTMENT OF DEFENSE. Retrieved June 25, 2021.
- Liebermann, Oren; Kaufman, Ellie; Cole, Devan (April 10, 2021). "Nearly 40% of Marines have declined Covid-19 vaccine". CNN. Retrieved April 11, 2021.
- "Secretary of Defense Mandates COVID-19 Vaccinations for Service Members". U.S. Department of Defense.
- "Pentagon mandates US military service members receive COVID vaccine immediately". www.wrcbtv.com.
- "Navy, Marine Corps Issue Policy, Deadlines for Mandatory COVID-19 Vaccinations for Active, Reserve Forces". September 2021.
- "94%: In Air Force, Vaccine Reluctance Falls Away as Deadline Approaches". September 29, 2021.
- "Army's plan for mandatory COVID-19 vaccines is here". September 14, 2021.
- "Vaccine passports underscore the necessity of U.S. privacy legislation" Brookings.edu. Retrieved 24 October 2021.
- Dai T, Song JS (September 2021). "Transforming COVID-19 vaccines into vaccination : Challenges and opportunities for management scientists". Health Care Management Science. 24 (3): 455–459. doi:10.1007/s10729-021-09563-3. PMC 8206869. PMID 34132977.
- "KFF COVID-19 Vaccine Monitor Dashboard". KFF. June 30, 2021. Retrieved July 1, 2021.
- Muñana, Cailey (December 15, 2020). "KFF COVID-19 Vaccine Monitor: December 2020". KFF. Retrieved November 22, 2021.
- ^ Dai, Tinglong; Song, Jing-Sheng (September 1, 2021). "Transforming COVID-19 vaccines into vaccination". Health Care Management Science. 24 (3): 455–459. doi:10.1007/s10729-021-09563-3. ISSN 1572-9389. PMC 8206869. PMID 34132977.
- "KFF COVID-19 Vaccine Monitor Dashboard". January 28, 2022.
- Romano, Andrew (May 22, 2020). "New Yahoo News/YouGov poll shows coronavirus conspiracy theories spreading on the right may hamper vaccine efforts". Yahoo News. Retrieved May 25, 2020.
- Murray, Patrick (April 14, 2021). "NATIONAL: ONE IN FIVE STILL SHUN VACCINE". Monmouth University. Retrieved April 18, 2021.
- "QU Poll Release Detail". QU Poll. Quinnipiac University. April 14, 2021. Retrieved April 19, 2021.
- Stolberg, Sheryl Gay; Karni, Annie (April 21, 2021). "Nation Faces 'Hand-to-Hand Combat' to Get Reluctant Americans Vaccinated". The New York Times. ISSN 0362-4331. Retrieved April 23, 2021.
- Lee, Thomas H.; Chen, Alice H. (January 27, 2021). "Last-Mile Logistics of Covid Vaccination — The Role of Health Care Organizations". New England Journal of Medicine. 384 (8): 685–687. doi:10.1056/NEJMp2100574. PMID 33503340. S2CID 231767335.
- "Poll: Vaccine Confidence Grows Despite J&J Pause". de Beaumont Foundation. April 20, 2021. Retrieved August 19, 2021.
- Bosman, Julie; Hoffman, Jan; Sanger-Katz, Margot; Arango, Tim (July 31, 2021). "Who Are the Unvaccinated in America? There's No One Answer". The New York Times. ISSN 0362-4331. Archived from the original on August 5, 2021. Retrieved August 13, 2021.
External links
| COVID-19 pandemic in the United States | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locations |
| ||||||||||||
| Responses |
| ||||||||||||
| Impacts |
| ||||||||||||
| Notable people |
| ||||||||||||
| COVID-19 vaccination in North America | |
|---|---|
| Sovereign states | |
| Dependencies and other territories | |