This is the current revision of this page, as edited by Fswitzer4 (talk | contribs) at 17:14, 18 August 2022 (Added UNII). The present address (URL) is a permanent link to this version.
Revision as of 17:14, 18 August 2022 by Fswitzer4 (talk | contribs) (Added UNII)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)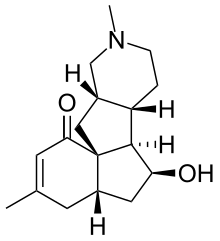
| |
| Names | |
|---|---|
| Preferred IUPAC name (4aS,6S,6aR,6bS,10aS,11aS)-6-Hydroxy-3,9-dimethyl-4,4a,5,6,6a,6b,7,8,9,10,10a,11-dodecahydro-1H-benzopentalenopyridin-1-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H25NO2 |
| Molar mass | 275.392 g·mol |
| Melting point | 165 to 166 °C (329 to 331 °F; 438 to 439 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
(−)-Magellanine is a member of the Lycopodium alkaloid class of natural products. It was isolated from the club moss Lycopodium magellanicum in 1976. It has been synthesized five times, with the first synthesis having been completed by the Larry E. Overman group at the University of California, Irvine in 1993. It has also been synthesized by the Leo Paquette group in 1993 at Ohio State University, the Chun-Chen Liao group in 2002 at National Tsing Hua University, the Miyuki Ishikazi and Tamiko Takahashi groups in 2005 at the Josai International University and Tokyo University of Science, and the Chisato Mukai group in 2007 at the Kanazawa University. One partial synthesis was completed by the A. I. Meyers group in 1995 at Colorado State University.
Biosynthetically, it is thought to have been derived from lysine. This was determined by conducting feeding studies of radiolabeled precursors.
References
- ^ Castillo, Mariano; Loyola, Luis A.; Morales, Glauco; Singh, Ishwar; Calvo, Crispin; Rolland, Herbert L.; MacLean, David B. (1976). "Isolation and Structure". Canadian Journal of Chemistry. 54 (18): 2893–2899. doi:10.1139/v76-409.
- Hirst, Gavin C. (1993). "First total synthesis of Lycopodium alkaloids of the magellanane group. Enantioselective total syntheses of (-)-magellanine and (+)-magellaninone". Journal of the American Chemical Society. 115 (7): 2992–2993. doi:10.1021/ja00060a064.
- Williams, John P. (1994). "Total Synthesis of the Lycopodium Alkaloids Magellanine and Magellaninone by Three-fold Annulation of 2-Cyclopentenone". Journal of the American Chemical Society. 116 (11): 4689–4696. doi:10.1021/ja00090a017.
- Yen, Chi-Feng (2002). "Concise and Efficient Total Synthesis of Lycopodium Alkaloid Magellanine". Angewandte Chemie International Edition. 41 (21): 4090–4093. doi:10.1002/1521-3773(20021104)41:21<4090::AID-ANIE4090>3.0.CO;2-#.
- Ishizaki, Miyuki; Niimi, Yuka; Hoshino, Osamu; Hara, Hiroshi; Takahashi, Tamiko (2005). "A formal total synthesis of Lycopodium alkaloid, (±)-magellanine, by using the intramolecular Pauson Khand reaction". Tetrahedron. 61 (16): 4053–4065. doi:10.1016/j.tet.2005.02.044.
- Kozaka, Takashi (2008). "ChemInform Abstract: Stereoselective Total Synthesis of Three Lycopodium Alkaloids, (-)-Magellanine (I), (+)-Magellaninone (II), and (+)-Paniculatine (III), Based on Two Pauson—Khand Reactions". ChemInform. 39 (18). doi:10.1002/chin.200818182.
- Meyers, A. I. "Partial Synthesis". J. Chem. Soc. Chem. Commun. 1995: 2511–2512.
- Ma, Xiaoqiang; Gang, David R. (2004). "Biosynthesis of the Lycopodium Alkaloids". Nat. Prod. Rep. 21: 752–772. doi:10.1039/b409720n.