This is an old revision of this page, as edited by Wretchskull (talk | contribs) at 10:24, 8 December 2022 (Reverted edits by 181.66.169.132 (talk) to last version by Stardustwk). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 10:24, 8 December 2022 by Wretchskull (talk | contribs) (Reverted edits by 181.66.169.132 (talk) to last version by Stardustwk)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Neurodevelopmental disorder "Attention Deficit" redirects here. For the album, see Attention Deficit (album). "ADD", "ADHD", and "Hyperactive" redirect here. For other uses, see ADD (disambiguation), ADHD (disambiguation), and Hyperactive (disambiguation).Medical condition
| Attention deficit hyperactivity disorder | |
|---|---|
 | |
| People with ADHD may struggle more than others to focus on tasks such as schoolwork, but can maintain an unusually intense level of attention for tasks they find rewarding or interesting. | |
| Specialty | |
| Symptoms | |
| Causes | Both genetic and environmental factors |
| Diagnostic method | Based on symptoms after other possible causes have been ruled out |
| Differential diagnosis | |
| Treatment |
|
| Medication | |
| Frequency | 84.7 million (2019, using DSM-IV-TR and ICD-10) |
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterised by excessive amounts of inattention, hyperactivity, and impulsivity that are pervasive, impairing in multiple contexts, and otherwise age-inappropriate.
ADHD symptoms arise from executive dysfunction, and emotional dysregulation is often considered a core symptom. In children, problems paying attention may result in poor school performance. ADHD is associated with other neurodevelopmental and mental disorders as well as some non-psychiatric disorders, which can cause additional impairment, especially in modern society. Although people with ADHD struggle to focus on tasks they are not particularly interested in completing, they are often able to maintain an unusually prolonged and intense level of attention for tasks they do find interesting or rewarding; this is known as hyperfocus.
The precise causes of ADHD are unknown in the majority of cases. Genetic factors play an important role; ADHD tends to run in families and has a heritability rate of 74%. Toxins and infections during pregnancy and brain damage may be environmental risks.
It affects about 5–7% of children when diagnosed via the DSM-IV criteria, and 1–2% when diagnosed via the ICD-10 criteria. Rates are similar between countries and differences in rates depend mostly on how it is diagnosed. ADHD is diagnosed approximately twice as often in boys than in girls, and 1.6 times more often in men than in women, although the disorder is overlooked in girls or diagnosed in later life because their symptoms sometimes differ from diagnostic criteria. About 30–50% of people diagnosed in childhood continue to have ADHD in adulthood, with 2.58% of adults estimated to have ADHD which began in childhood. In adults, hyperactivity is usually replaced by inner restlessness, and adults often develop coping skills to compensate for their impairments. The condition can be difficult to tell apart from other conditions, as well as from high levels of activity within the range of normal behavior. ADHD has a negative impact on patients’ health related quality of life and that this may be further exacerbated by, or may increase the risk of, other psychiatric conditions such as anxiety and depression.
ADHD management recommendations vary and usually involve some combination of medications, counseling, and lifestyle changes. The British guideline emphasises environmental modifications and education for individuals and carers about ADHD as the first response. If symptoms persist, parent-training, medication, or psychotherapy (especially cognitive behavioral therapy) can be recommended based on age. Canadian and American guidelines recommend medications and behavioral therapy together, except in preschool-aged children for whom the first-line treatment is behavioral therapy alone. Stimulant medications are the most effective pharmaceutical treatment, although there may be side effects and any improvements will be reverted if medication is ceased.
ADHD, its diagnosis, and its treatment have been considered controversial since the 1970s. These controversies have involved doctors, teachers, policymakers, parents, and the media. Topics have included causes of ADHD and the use of stimulant medications in its treatment. ADHD is now a well-validated clinical diagnosis in children and adults, and the debate in the scientific community mainly centers on how it is diagnosed and treated. ADHD was officially known as attention deficit disorder (ADD) from 1980 to 1987; prior to the 1980s, it was known as hyperkinetic reaction of childhood. Symptoms similar to those of ADHD have been described in medical literature dating back to the 18th century.
Signs and symptoms
Inattention, hyperactivity (restlessness in adults), disruptive behavior, and impulsivity are common in ADHD. Academic difficulties are frequent as are problems with relationships. The symptoms can be difficult to define, as it is hard to draw a line at where normal levels of inattention, hyperactivity, and impulsivity end and significant levels requiring interventions begin.
According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and its text revision (DSM-5-TR), symptoms must be present for six months or more to a degree that is much greater than others of the same age. This requires at least six symptoms of either inattention or hyperactivity/impulsivity for those under 17 and at least five symptoms for those 17 years or older. The symptoms must be present in at least two settings (e.g., social, school, work, or home), and must directly interfere with or reduce quality of functioning. Additionally, several symptoms must have been present before age twelve.
Subtypes
ADHD is divided into three primary presentations:
- predominantly inattentive (ADHD-PI or ADHD-I)
- predominantly hyperactive-impulsive (ADHD-PH or ADHD-HI)
- combined type (ADHD-C).
The table "Symptoms" lists the symptoms for ADHD-I and ADHD-HI from two major classification systems. Symptoms which can be better explained by another psychiatric or medical condition which an individual has are not considered to be a symptom of ADHD for that person.
| Presentations | DSM-5 and DSM-5-TR symptoms | ICD-11 symptoms |
|---|---|---|
| Inattention | Six or more of the following symptoms in children, and five or more in adults, excluding situations where these symptoms are better explained by another psychiatric or medical condition:
|
Multiple symptoms of inattention that directly negatively impact occupational, academic or social functioning. Symptoms may not be present when engaged in highly stimulating tasks with frequent rewards. Symptoms are generally from the following clusters:
The individual may also meet the criteria for hyperactivity-impulsivity, but the inattentive symptoms are predominant. |
| Hyperactivity-Impulsivity | Six or more of the following symptoms in children, and five or more in adults, excluding situations where these symptoms are better explained by another psychiatric or medical condition:
|
Multiple symptoms of hyperactivity/impulsivity that directly negatively impact occupational, academic or social functioning. Typically, these tend to be most apparent in environments with structure or which require self-control. Symptoms are generally from the following clusters:
The individual may also meet the criteria for inattention, but the hyperactive-impulsive symptoms are predominant. |
| Combined | Meet the criteria for both inattentive and hyperactive-impulsive ADHD. | Criteria are met for both inattentive and hyperactive-impulsive ADHD, with neither clearly predominating. |
Girls and women with ADHD tend to display fewer hyperactivity and impulsivity symptoms but more symptoms of inattention and distractibility.
Symptoms are expressed differently and more subtly as the individual ages. Hyperactivity tends to become less overt with age and turns into inner restlessness, difficulty relaxing or remaining still, talkativeness or constant mental activity in teens and adults with ADHD. Impulsivity in adulthood may appear as thoughtless behaviour, impatience, irresponsible spending and sensation-seeking behaviours, while inattention may appear as becoming easily bored, difficulty with organization, remaining on task and making decisions, and sensitivity to stress.
Although not listed as an official symptom for this condition, emotional dysregulation or mood lability is generally understood to be a common symptom of ADHD. People with ADHD of all ages are more likely to have problems with social skills, such as social interaction and forming and maintaining friendships. This is true for all presentations. About half of children and adolescents with ADHD experience social rejection by their peers compared to 10–15% of non-ADHD children and adolescents. People with attention deficits are prone to having difficulty processing verbal and nonverbal language which can negatively affect social interaction. They also may drift off during conversations, miss social cues, and have trouble learning social skills.
Difficulties managing anger are more common in children with ADHD as are delays in speech, language and motor development. Poorer handwriting is more common in children with ADHD. Poor handwriting in many situations can be a side effect of ADHD in itself due to decreased attentiveness but when it's a constant problem it may also be in part due to both Dyslexic and Dysgraphic individuals having higher rates of ADHD than the general population, with 3 in 10 people who have dyslexia also having ADHD. Although it causes significant difficulty, many children with ADHD have an attention span equal to or greater than that of other children for tasks and subjects they find interesting.
Comorbidities
Psychiatric
In children, ADHD occurs with other disorders about two-thirds of the time.
Other neurodevelopmental conditions are common comorbidities. Autism spectrum disorder (ASD), co-occurring at a rate of 21% in those with ADHD, affects social skills, ability to communicate, behaviour, and interests. Both ADHD and ASD can be diagnosed in the same person. Learning disabilities have been found to occur in about 20–30% of children with ADHD. Learning disabilities can include developmental speech and language disorders, and academic skills disorders. ADHD, however, is not considered a learning disability, but it very frequently causes academic difficulties. Intellectual disabilities and Tourette's syndrome are also common.
ADHD is often comorbid with disruptive, impulse control, and conduct disorders. Oppositional defiant disorder (ODD) occurs in about 25% of children with an inattentive presentation and 50% of those with a combined presentation. It is characterised by angry or irritable mood, argumentative or defiant behavior and vindictiveness which are age-inappropriate. Conduct disorder (CD) occurs in about 25% of adolescents with ADHD. It is characterised by aggression, destruction of property, deceitfulness, theft and violations of rules. Adolescents with ADHD who also have CD are more likely to develop antisocial personality disorder in adulthood. Brain imaging supports that CD and ADHD are separate conditions, wherein conduct disorder was shown to reduce the size of one's temporal lobe and limbic system, and increase the size of one's orbitofrontal cortex, whereas ADHD was shown to reduce connections in the cerebellum and prefrontal cortex more broadly. Conduct disorder involves more impairment in motivation control than ADHD. Intermittent explosive disorder is characterised by sudden and disproportionate outbursts of anger and co-occurs in individuals with ADHD more frequently than in the general population.
Anxiety and mood disorders are frequent comorbidities. Anxiety disorders have been found to occur more commonly in the ADHD population, as have mood disorders (especially bipolar disorder and major depressive disorder). Boys diagnosed with the combined ADHD subtype are more likely to have a mood disorder. Adults and children with ADHD sometimes also have bipolar disorder, which requires careful assessment to accurately diagnose and treat both conditions.
Sleep disorders and ADHD commonly co-exist. They can also occur as a side effect of medications used to treat ADHD. In children with ADHD, insomnia is the most common sleep disorder with behavioral therapy being the preferred treatment. Problems with sleep initiation are common among individuals with ADHD but often they will be deep sleepers and have significant difficulty getting up in the morning. Melatonin is sometimes used in children who have sleep onset insomnia. Specifically, the sleep disorder restless legs syndrome has been found to be more common in those with ADHD and is often due to iron deficiency anemia. However, restless legs can simply be a part of ADHD and requires careful assessment to differentiate between the two disorders. Delayed sleep phase disorder is also a common comorbidity of those with ADHD.
There are other psychiatric conditions which are often co-morbid with ADHD, such as substance use disorders. Individuals with ADHD are at increased risk of substance abuse. This is most commonly seen with alcohol or cannabis. The reason for this may be an altered reward pathway in the brains of ADHD individuals, self-treatment and increased psychosocial risk factors. This makes the evaluation and treatment of ADHD more difficult, with serious substance misuse problems usually treated first due to their greater risks. Other psychiatric conditions include reactive attachment disorder, characterised by a severe inability to appropriately relate socially, and sluggish cognitive tempo, a cluster of symptoms that potentially comprises another attention disorder and may occur in 30–50% of ADHD cases, regardless of the subtype. Individuals with ADHD are 4x more likely to develop and be diagnosed with an eating disorder (Anorexia, Bulimia, Binge Eating, ARFID) compared to those without ADHD. Individuals with diagnosed eating disorders are 2.6x more likely to have ADHD than those without eating disorders, though these numbers are likely much lower than actual rates, due to limitations with screening and diagnosis in marginalized populations.
Trauma
ADHD, trauma, and Adverse Childhood Experiences are also comorbid, which could in part be potentially explained by the similarity in presentation between different diagnoses. The symptoms of ADHD and PTSD can have significant behavioral overlap with ADHD—in particular, motor restlessness, difficulty concentrating, distractibility, irritability/anger, emotional constriction or dysregulation, poor impulse control, and forgetfulness are common in both. This could result in trauma-related disorders or ADHD being mis-identified as the other. Additionally, traumatic events in childhood are a risk factor for ADHD - it can lead to structural brain changes and the development of ADHD behaviors. Finally, the behavioral consequences of ADHD symptoms cause a higher chance of the individual experiencing trauma (and therefore ADHD leads to a concrete diagnosis of a trauma-related disorder).
Non-psychiatric
Some non-psychiatric conditions are also comorbidities of ADHD. This includes epilepsy, a neurological condition characterised by recurrent seizures. There are well established associations between ADHD and obesity, asthma and sleep disorders, and an association with celiac disease. Children with ADHD have a higher risk for migraine headaches, but have no increased risk of tension-type headaches. In addition, children with ADHD may also experience headaches as a result of medication.
A 2021 review reported that several neurometabolic disorders caused by inborn errors of metabolism converge on common neurochemical mechanisms that interfere with biological mechanisms also considered central in ADHD pathophysiology and treatment. This highlights the importance of close collaboration between health services to avoid clinical overshadowing.
Suicide risk
Systematic reviews conducted in 2017 and 2020 found strong evidence that ADHD is associated with increased suicide risk across all age groups, as well as growing evidence that an ADHD diagnosis in childhood or adolescence represents a significant future suicidal risk factor. Potential causes include ADHD's association with functional impairment, negative social, educational and occupational outcomes, and financial distress. A 2019 meta-analysis indicated a significant association between ADHD and suicidal spectrum behaviors (suicidal attempts, ideations, plans, and completed suicides); across the studies examined, the prevalence of suicide attempts in individuals with ADHD was 18.9%, compared to 9.3% in individuals without ADHD, and the findings were substantially replicated among studies which adjusted for other variables. However, the relationship between ADHD and suicidal spectrum behaviors remains unclear due to mixed findings across individual studies and the complicating impact of comorbid psychiatric disorders. There is no clear data on whether there is a direct relationship between ADHD and suicidality, or whether ADHD increases suicide risk through comorbidities.
IQ test performance
Certain studies have found that people with ADHD tend to have lower scores on intelligence quotient (IQ) tests. The significance of this is controversial due to the differences between people with ADHD and the difficulty determining the influence of symptoms, such as distractibility, on lower scores rather than intellectual capacity. In studies of ADHD, higher IQs may be over-represented because many studies exclude individuals who have lower IQs despite those with ADHD scoring on average nine points lower on standardized intelligence measures. In individuals with high intelligence, there is increased risk of a missed ADHD diagnosis, possibly because of compensatory strategies in highly intelligent individuals.
Studies of adults suggest that negative differences in intelligence are not meaningful and may be explained by associated health problems.
Causes
ADHD is generally claimed to be the result of neurological dysfunction in processes associated with the production or use of dopamine and norepinephrine in various brain structures, but there are no confirmed causes. It may involve interactions between genetics and the environment.
Genetics
ADHD has a high heritability of 74%, meaning that 74% of the presence of ADHD in the population is due to genetic factors. There are multiple gene variants which each slightly increase the likelihood of a person having ADHD; it is polygenic and arises through the combination of many gene variants which each have a small effect. The siblings of children with ADHD are three to four times more likely to develop the disorder than siblings of children without the disorder.
Arousal is related to dopaminergic functioning, and ADHD presents with low dopaminergic functioning. Typically, a number of genes are involved, many of which directly affect dopamine neurotransmission. Those involved with dopamine include DAT, DRD4, DRD5, TAAR1, MAOA, COMT, and DBH. Other genes associated with ADHD include SERT, HTR1B, SNAP25, GRIN2A, ADRA2A, TPH2, and BDNF. A common variant of a gene called latrophilin 3 is estimated to be responsible for about 9% of cases and when this variant is present, people are particularly responsive to stimulant medication. The 7 repeat variant of dopamine receptor D4 (DRD4–7R) causes increased inhibitory effects induced by dopamine and is associated with ADHD. The DRD4 receptor is a G protein-coupled receptor that inhibits adenylyl cyclase. The DRD4–7R mutation results in a wide range of behavioral phenotypes, including ADHD symptoms reflecting split attention. The DRD4 gene is both linked to novelty seeking and ADHD. The genes GFOD1 and CDH13 show strong genetic associations with ADHD. CHD13's association with ASD, schizophrenia, bipolar disorder, and depression make it an interesting candidate causative gene. Another candidate causative gene that has been identified is ADGRL3. In zebrafish, knockout of this gene causes a loss of dopaminergic function in the ventral diencephalon and the fish display a hyperactive/impulsive phenotype.
For genetic variation to be used as a tool for diagnosis, more validating studies need to be performed. However, smaller studies have shown that genetic polymorphisms in genes related to catecholaminergic neurotransmission or the SNARE complex of the synapse can reliably predict a person's response to stimulant medication. Rare genetic variants show more relevant clinical significance as their penetrance (the chance of developing the disorder) tends to be much higher. However their usefulness as tools for diagnosis is limited as no single gene predicts ADHD. ASD shows genetic overlap with ADHD at both common and rare levels of genetic variation.
Environment
In addition to genetics, some environmental factors might play a role in causing ADHD. Alcohol intake during pregnancy can cause fetal alcohol spectrum disorders which can include ADHD or symptoms like it. Children exposed to certain toxic substances, such as lead or polychlorinated biphenyls, may develop problems which resemble ADHD. Exposure to the organophosphate insecticides chlorpyrifos and dialkyl phosphate is associated with an increased risk; however, the evidence is not conclusive. Exposure to tobacco smoke during pregnancy can cause problems with central nervous system development and can increase the risk of ADHD. Nicotine exposure during pregnancy may be an environmental risk.
Extreme premature birth, very low birth weight, and extreme neglect, abuse, or social deprivation also increase the risk as do certain infections during pregnancy, at birth, and in early childhood. These infections include, among others, various viruses (measles, varicella zoster encephalitis, rubella, enterovirus 71). At least 30% of children with a traumatic brain injury later develop ADHD and about 5% of cases are due to brain damage.
Some studies suggest that in a small number of children, artificial food dyes or preservatives may be associated with an increased prevalence of ADHD or ADHD-like symptoms, but the evidence is weak and may only apply to children with food sensitivities. The European Union has put in place regulatory measures based on these concerns. In a minority of children, intolerances or allergies to certain foods may worsen ADHD symptoms.
Individuals with hypokalemic sensory overstimulation are sometimes diagnosed as having attention deficit hyperactivity disorder (ADHD), raising the possibility that a subtype of ADHD has a cause that can be understood mechanistically and treated in a novel way. The sensory overload is treatable with oral potassium gluconate.
Research does not support popular beliefs that ADHD is caused by eating too much refined sugar, watching too much television, parenting, poverty or family chaos; however, they might worsen ADHD symptoms in certain people.
Society
The youngest children in a class have been found to be more likely to be diagnosed as having ADHD, possibly due to them being developmentally behind their older classmates. They also appear to use ADHD medications at nearly twice the rate of their peers.
In some cases, an inappropriate diagnosis of ADHD may reflect a dysfunctional family or a poor educational system, rather than any true presence of ADHD in the individual. In other cases, it may be explained by increasing academic expectations, with a diagnosis being a method for parents in some countries to get extra financial and educational support for their child. Behaviors typical of ADHD occur more commonly in children who have experienced violence and emotional abuse.
Pathophysiology
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems, particularly those involving dopamine and norepinephrine. The dopamine and norepinephrine pathways that originate in the ventral tegmental area and locus coeruleus project to diverse regions of the brain and govern a variety of cognitive processes. The dopamine pathways and norepinephrine pathways which project to the prefrontal cortex and striatum are directly responsible for modulating executive function (cognitive control of behavior), motivation, reward perception, and motor function; these pathways are known to play a central role in the pathophysiology of ADHD. Larger models of ADHD with additional pathways have been proposed.
Brain structure

In children with ADHD, there is a general reduction of volume in certain brain structures, with a proportionally greater decrease in the volume in the left-sided prefrontal cortex. The posterior parietal cortex also shows thinning in individuals with ADHD compared to controls. Other brain structures in the prefrontal-striatal-cerebellar and prefrontal-striatal-thalamic circuits have also been found to differ between people with and without ADHD.
The subcortical volumes of the accumbens, amygdala, caudate, hippocampus, and putamen appears smaller in individuals with ADHD compared with controls. Structural MRI studies have also revealed differences in white matter, with marked differences in inter-hemispheric asymmetry between ADHD and typically developing youths
Functional MRI fMRI studies have revealed a number of differences between ADHD and control brains. Independent component analysis performed on resting-state fMRI data have revealed that individuals with the inattentive type of ADHD have significantly more independent components are required to describe the variance of this data.
Neurotransmitter pathways
Previously, it had been suggested that the elevated number of dopamine transporters in people with ADHD was part of the pathophysiology, but it appears the elevated numbers may be due to adaptation following exposure to stimulant medication. Current models involve the mesocorticolimbic dopamine pathway and the locus coeruleus-noradrenergic system. ADHD psychostimulants possess treatment efficacy because they increase neurotransmitter activity in these systems. There may additionally be abnormalities in serotonergic, glutamatergic, or cholinergic pathways.
Executive function and motivation
The symptoms of ADHD arise from a deficiency in certain executive functions (e.g., attentional control, inhibitory control, and working memory). Executive functions are a set of cognitive processes that are required to successfully select and monitor behaviors that facilitate the attainment of one's chosen goals. The executive function impairments that occur in ADHD individuals result in problems with staying organised, time keeping, excessive procrastination, maintaining concentration, paying attention, ignoring distractions, regulating emotions, and remembering details. People with ADHD appear to have unimpaired long-term memory, and deficits in long-term recall appear to be attributed to impairments in working memory. Due to the rates of brain maturation and the increasing demands for executive control as a person gets older, ADHD impairments may not fully manifest themselves until adolescence or even early adulthood.
ADHD has also been associated with motivational deficits in children. Children with ADHD often find it difficult to focus on long-term over short-term rewards, and exhibit impulsive behavior for short-term rewards.
Paradoxical reaction to neuroactive substances
Another sign of the structurally altered signal processing in the central nervous system in this group of people is the conspicuously common Paradoxical reaction (ca. 10–20 % of patients). These are unexpected reactions in the opposite direction as with a normal effect, or otherwise significant different reactions. These are reactions to neuroactive substances such as local anesthetic at the dentist, sedative, caffeine, antihistamine, weak neuroleptics and central and peripheral painkillers. Since the causes of paradoxical reactions are at least partly genetic, it may be useful in critical situations, for example before operations, to ask whether such abnormalities may also exist in family members.
Diagnosis
ADHD is diagnosed by an assessment of a person's behavioral and mental development, including ruling out the effects of drugs, medications, and other medical or psychiatric problems as explanations for the symptoms. ADHD diagnosis often takes into account feedback from parents and teachers with most diagnoses begun after a teacher raises concerns. It may be viewed as the extreme end of one or more continuous human traits found in all people. Imaging studies of the brain do not give consistent results between individuals; thus, they are only used for research purposes and not a diagnosis.
In North America and Australia, DSM-5 criteria are used for diagnosis, while European countries usually use the ICD-10. The DSM-IV criteria for diagnosis of ADHD is 3–4 times more likely to diagnose ADHD than is the ICD-10 criteria. ADHD is alternately classified as neurodevelopmental disorder or a disruptive behavior disorder along with ODD, CD, and antisocial personality disorder. A diagnosis does not imply a neurological disorder.
Associated conditions that should be screened for include anxiety, depression, ODD, CD, and learning and language disorders. Other conditions that should be considered are other neurodevelopmental disorders, tics, and sleep apnea.
Self-rating scales, such as the ADHD rating scale and the Vanderbilt ADHD diagnostic rating scale, are used in the screening and evaluation of ADHD. Electroencephalography is not accurate enough to make an ADHD diagnosis.
Classification
Diagnostic and Statistical Manual
As with many other psychiatric disorders, a formal diagnosis should be made by a qualified professional based on a set number of criteria. In the United States, these criteria are defined by the American Psychiatric Association in the DSM. Based on the DSM-5 criteria published in 2013 and the DSM-5-TR criteria published in 2022, there are three presentations of ADHD:
- ADHD, predominantly inattentive type, presents with symptoms including being easily distracted, forgetful, daydreaming, disorganization, poor concentration, and difficulty completing tasks.
- ADHD, predominantly hyperactive-impulsive type, presents with excessive fidgeting and restlessness, hyperactivity, and difficulty waiting and remaining seated.
- ADHD, combined type, is a combination of the first two presentations.
This subdivision is based on presence of at least six (in children) or five (in older teenagers and adults) out of nine long-term (lasting at least six months) symptoms of inattention, hyperactivity–impulsivity, or both. To be considered, several symptoms must have appeared by the age of six to twelve and occur in more than one environment (e.g. at home and at school or work). The symptoms must be inappropriate for a child of that age and there must be clear evidence that they are causing social, school or work related problems.
The DSM-5 and the DSM-5-TR also provide two diagnoses for individuals who have symptoms of ADHD but do not entirely meet the requirements. Other Specified ADHD allows the clinician to describe why the individual does not meet the criteria, whereas Unspecified ADHD is used where the clinician chooses not to describe the reason.
International Classification of Diseases
In the eleventh revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-11) by the World Health Organization, the disorder is classified as Attention deficit hyperactivity disorder (with the code 6A05). The defined subtypes are similar to those of the DSM-5: predominantly inattentive presentation (6A05.0); predominantly hyperactive-impulsive presentation(6A05.1); combined presentation (6A05.2). However, the ICD-11 includes two residual categories for individuals who do not entirely match any of the defined subtypes: other specified presentation (6A05.Y) where the clinician includes detail on the individual's presentation; and presentation unspecified (6A05.Z) where the clinician does not provide detail.
In the tenth revision (ICD-10), the symptoms of hyperkinetic disorder were analogous to ADHD in the ICD-11. When a conduct disorder (as defined by ICD-10) is present, the condition was referred to as hyperkinetic conduct disorder. Otherwise, the disorder was classified as disturbance of activity and attention, other hyperkinetic disorders or hyperkinetic disorders, unspecified. The latter was sometimes referred to as hyperkinetic syndrome.
Social construct theory
The social construct theory of ADHD suggests that, because the boundaries between normal and abnormal behavior are socially constructed (i.e. jointly created and validated by all members of society, and in particular by physicians, parents, teachers, and others), it then follows that subjective valuations and judgements determine which diagnostic criteria are used and thus, the number of people affected. This difference means using DSM-IV criteria could diagnose ADHD at rates three to four times higher than ICD-10 criteria. Thomas Szasz, a supporter of this theory, has argued that ADHD was "invented and then given a name".
Adults
Main article: Adult attention deficit hyperactivity disorderAdults with ADHD are diagnosed under the same criteria, including that their signs must have been present by the age of six to twelve. The individual is the best source for information in diagnosis, however others may provide useful information about the individual's symptoms currently and in childhood; a family history of ADHD also adds weight to a diagnosis. While the core symptoms of ADHD are similar in children and adults, they often present differently in adults than in children: for example, excessive physical activity seen in children may present as feelings of restlessness and constant mental activity in adults.
Worldwide, it is estimated that 2.58% of adults have persistent ADHD (where the individual currently meets the criteria and there is evidence of childhood onset), and 6.76% of adults have symptomatic ADHD (meaning that they currently meet the criteria for ADHD, regardless of childhood onset). In 2020, this was 139.84 million and 366.33 million affected adults respectively. Around 15% of children with ADHD continue to meet full DSM-IV-TR criteria at 25 years of age, and 50% still experience some symptoms. As of 2010, most adults remain untreated. Many adults with ADHD without diagnosis and treatment have a disorganised life, and some use non-prescribed drugs or alcohol as a coping mechanism. Other problems may include relationship and job difficulties, and an increased risk of criminal activities. Associated mental health problems include depression, anxiety disorders, and learning disabilities.
Some ADHD symptoms in adults differ from those seen in children. While children with ADHD may climb and run about excessively, adults may experience an inability to relax, or may talk excessively in social situations. Adults with ADHD may start relationships impulsively, display sensation-seeking behavior, and be short-tempered. Addictive behavior such as substance abuse and gambling are common. This led to those who presented differently as they aged having outgrown the DSM-IV criteria. The DSM-5 criteria does specifically deal with adults unlike that of DSM-IV, which does not fully take into account the differences in impairments seen in adulthood compared to childhood.
For diagnosis in an adult, having symptoms since childhood is required. Nevertheless, a proportion of adults who meet the criteria for ADHD in adulthood would not have been diagnosed with ADHD as children. Most cases of late-onset ADHD develop the disorder between the ages of 12-16 and may therefore be considered early adult or adolescent-onset ADHD.
Differential diagnosis
| Depression disorder | Anxiety disorder | Bipolar disorder |
|---|---|---|
|
|
in manic state
in depressive state
|
The DSM provides potential differential diagnoses - potential alternate explanations for specific symptoms. Assessment and investigation of clinical history determines which is the most appropriate diagnosis. The DSM-5 suggests ODD, intermittent explosive disorder, and other neurodevelopmental disorders (such as stereotypic movement disorder and Tourette's disorder), in addition to specific learning disorder, intellectual developmental disorder, ASD, reactive attachment disorder, anxiety disorders, depressive disorders, bipolar disorder, disruptive mood dysregulation disorder, substance use disorder, personality disorders, psychotic disorders, medication-induced symptoms, and neurocognitive disorders. Many but not all of these are also common comorbidities of ADHD. The DSM-5-TR also suggests post-traumatic stress disorder.
Symptoms of ADHD, such as low mood and poor self-image, mood swings, and irritability, can be confused with dysthymia, cyclothymia or bipolar disorder as well as with borderline personality disorder. Some symptoms that are due to anxiety disorders, personality disorder, developmental disabilities or intellectual disability or the effects of substance abuse such as intoxication and withdrawal can overlap with ADHD. These disorders can also sometimes occur along with ADHD. Medical conditions which can cause ADHD-type symptoms include: hyperthyroidism, seizure disorder, lead toxicity, hearing deficits, hepatic disease, sleep apnea, drug interactions, untreated celiac disease, and head injury.
Primary sleep disorders may affect attention and behavior and the symptoms of ADHD may affect sleep. It is thus recommended that children with ADHD be regularly assessed for sleep problems. Sleepiness in children may result in symptoms ranging from the classic ones of yawning and rubbing the eyes, to hyperactivity and inattentiveness. Obstructive sleep apnea can also cause ADHD-type symptoms.
Management
Main article: Attention deficit hyperactivity disorder managementThe management of ADHD typically involves counseling or medications, either alone or in combination. While treatment may improve long-term outcomes, it does not get rid of negative outcomes entirely. Medications used include stimulants, atomoxetine, alpha-2 adrenergic receptor agonists, and sometimes antidepressants. In those who have trouble focusing on long-term rewards, a large amount of positive reinforcement improves task performance. ADHD stimulants also improve persistence and task performance in children with ADHD. "Recent evidence from observational and registry studies indicates that pharmacological treatment of ADHD is associated with increased achievement and decreased absenteeism at school, a reduced risk of trauma-related emergency hospital visits, reduced risks of suicide and attempted suicide, and decreased rates of substance abuse and criminality".
Behavioral therapies
There is good evidence for the use of behavioral therapies in ADHD. They are the recommended first-line treatment in those who have mild symptoms or who are preschool-aged. Psychological therapies used include: psychoeducational input, behavior therapy, cognitive behavioral therapy, interpersonal psychotherapy, family therapy, school-based interventions, social skills training, behavioral peer intervention, organization training, and parent management training. Neurofeedback has greater treatment effects than non-active controls for up to 6 months and possibly a year following treatment, and may have treatment effects comparable to active controls (controls proven to have a clinical effect) over that time period. Despite efficacy in research, there is insufficient regulation of neurofeedback practice, leading to ineffective applications and false claims regarding innovations. Parent training may improve a number of behavioral problems including oppositional and non-compliant behaviors.
There is little high-quality research on the effectiveness of family therapy for ADHD—but the existing evidence shows that it is similar to community care, and better than placebo. ADHD-specific support groups can provide information and may help families cope with ADHD.
Social skills training, behavioral modification, and medication may have some limited beneficial effects in peer relationships. Stable, high-quality friendships with non-deviant peers protect against later psychological problems.
Medication
Stimulants
Methylphenidate and amphetamine or its derivatives are first-line treatments for ADHD as they are considered the most effective pharmaceutical treatments. About 70 percent respond to the first stimulant tried and as few as 10 percent respond to neither amphetamines nor methylphenidate. Stimulants may also reduce the risk of unintentional injuries in children with ADHD. Magnetic resonance imaging studies suggest that long-term treatment with amphetamine or methylphenidate decreases abnormalities in brain structure and function found in subjects with ADHD. A 2018 review found the greatest short-term benefit with methylphenidate in children, and amphetamines in adults.
The likelihood of developing insomnia for ADHD patients taking stimulants has been measured at between 11 and 45 percent for different medications, and may be a main reason for discontinuation. Other side effects, such as tics, decreased appetite and weight loss, or emotional lability, may also lead to discontinuation. Stimulant psychosis and mania are rare at therapeutic doses, appearing to occur in approximately 0.1% of individuals, within the first several weeks after starting amphetamine therapy. The safety of these medications in pregnancy is unclear. Symptom improvement is not sustained if medication is ceased.
The long-term effects of ADHD medication have yet to be fully determined, although stimulants are generally beneficial and safe for up to two years for children and adolescents. Regular monitoring has been recommended in those on long-term treatment. There are indications suggesting that stimulant therapy for children and adolescents should be stopped periodically to assess continuing need for medication, decrease possible growth delay, and reduce tolerance. Although potentially addictive at high doses, stimulants used to treat ADHD have low potential for abuse. Treatment with stimulants is either protective against substance abuse or has no effect.
The majority of studies on nicotine and other nicotinic agonists as treatments for ADHD have shown favorable results; however, no nicotinic drug has been approved for ADHD treatment. Caffeine was formerly used as a second-line treatment for ADHD. It is considered less effective than methylphenidate or amphetamine but more so than placebo for children with ADHD. Pseudoephedrine and ephedrine do not affect ADHD symptoms.
Modafinil has shown some efficacy in reducing the severity of ADHD in children and adolescents. It may be prescribed off-label to treat ADHD.
Non-stimulants
There are a number of non-stimulant medications, such as Viloxazine, atomoxetine, bupropion, guanfacine, amantadine (effective in children and adolescents but still not been seen for adults), and clonidine, that may be used as alternatives, or added to stimulant therapy. There are no good studies comparing the various medications; however, they appear more or less equal with respect to side effects. For children, stimulants appear to improve academic performance while atomoxetine does not.
Atomoxetine, due to its lack of addiction liability, may be preferred in those who are at risk of recreational or compulsive stimulant use, although evidence is lacking to support its use over stimulants for this reason. Evidence supports its ability to improve symptoms when compared to placebo.
Amantadine was shown to induce similar improvements in children treated methylphenidate, with less frequent side effects. A 2021 retrospective study showed showed that amantadine may serve as an effective adjunct to stimulants for ADHD–related symptoms and appears to be a safer alternative to second- or third-generation antipsychotics.
There is little evidence on the effects of medication on social behaviors. Antipsychotics may also be used to treat aggression in ADHD.
Guidelines
Guidelines on when to use medications vary by country. The United Kingdom's National Institute for Health and Care Excellence recommends use for children only in severe cases, though for adults medication is a first-line treatment. Conversely, most United States guidelines recommend medications in most age groups. Medications are especially not recommended for preschool children. Underdosing of stimulants can occur, and can result in a lack of response or later loss of effectiveness. This is particularly common in adolescents and adults as approved dosing is based on school-aged children, causing some practitioners to use weight-based or benefit-based off-label dosing instead.
Exercise
Regular physical exercise, particularly aerobic exercise, is an effective add-on treatment for ADHD in children and adults, particularly when combined with stimulant medication (although the best intensity and type of aerobic exercise for improving symptoms are not currently known). The long-term effects of regular aerobic exercise in ADHD individuals include better behavior and motor abilities, improved executive functions (including attention, inhibitory control, and planning, among other cognitive domains), faster information processing speed, and better memory. Parent-teacher ratings of behavioral and socio-emotional outcomes in response to regular aerobic exercise include: better overall function, reduced ADHD symptoms, better self-esteem, reduced levels of anxiety and depression, fewer somatic complaints, better academic and classroom behavior, and improved social behavior. Exercising while on stimulant medication augments the effect of stimulant medication on executive function. It is believed that these short-term effects of exercise are mediated by an increased abundance of synaptic dopamine and norepinephrine in the brain.
Diet
Dietary modifications are not recommended as of 2019 by the American Academy of Pediatrics, the National Institute for Health and Care Excellence, or the Agency for Healthcare Research and Quality due to insufficient evidence. A 2013 meta-analysis found less than a third of children with ADHD see some improvement in symptoms with free fatty acid supplementation or decreased eating of artificial food coloring. These benefits may be limited to children with food sensitivities or those who are simultaneously being treated with ADHD medications. This review also found that evidence does not support removing other foods from the diet to treat ADHD. A 2014 review found that an elimination diet results in a small overall benefit in a minority of children, such as those with allergies. A 2016 review stated that the use of a gluten-free diet as standard ADHD treatment is not advised. A 2017 review showed that a few-foods elimination diet may help children too young to be medicated or not responding to medication, while free fatty acid supplementation or decreased eating of artificial food coloring as standard ADHD treatment is not advised. Chronic deficiencies of iron, magnesium and iodine may have a negative impact on ADHD symptoms. There is a small amount of evidence that lower tissue zinc levels may be associated with ADHD. In the absence of a demonstrated zinc deficiency (which is rare outside of developing countries), zinc supplementation is not recommended as treatment for ADHD. However, zinc supplementation may reduce the minimum effective dose of amphetamine when it is used with amphetamine for the treatment of ADHD.
Prognosis
ADHD persists into adulthood in about 30–50% of cases. Those affected are likely to develop coping mechanisms as they mature, thus compensating to some extent for their previous symptoms. Children with ADHD have a higher risk of unintentional injuries. Effects of medication on functional impairment and quality of life (e.g. reduced risk of accidents) have been found across multiple domains. Rates of smoking among those with ADHD are higher than in the general population at about 40%.
Individuals with ADHD are significantly overrepresented in prison populations. Although there is no generally accepted estimate of ADHD prevalence among inmates, a 2015 meta-analysis estimated a prevalence of 25.5%, and a larger 2018 meta-analysis estimated the frequency to be 26.2%. ADHD is more common among longer-term inmates; a 2010 study at Norrtälje Prison, a high-security prison in Sweden, found an estimated ADHD prevalence of 40%.
Epidemiology
Main article: Epidemiology of attention deficit hyperactive disorder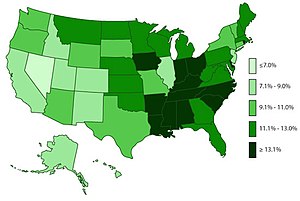
ADHD is estimated to affect about 6–7% of people aged 18 and under when diagnosed via the DSM-IV criteria. When diagnosed via the ICD-10 criteria, rates in this age group are estimated around 1–2%. Children in North America appear to have a higher rate of ADHD than children in Africa and the Middle East; this is believed to be due to differing methods of diagnosis rather than a difference in underlying frequency. As of 2019, it was estimated to affect 84.7 million people globally. If the same diagnostic methods are used, the rates are similar between countries. ADHD is diagnosed approximately three times more often in boys than in girls. This may reflect either a true difference in underlying rate, or that women and girls with ADHD are less likely to be diagnosed.
Rates of diagnosis and treatment have increased in both the United Kingdom and the United States since the 1970s. Prior to 1970, it was rare for children to be diagnosed with ADHD, while in the 1970s rates were about 1%. This is believed to be primarily due to changes in how the condition is diagnosed and how readily people are willing to treat it with medications rather than a true change in how common the condition is. It was believed changes to the diagnostic criteria in 2013 with the release of the DSM-5 would increase the percentage of people diagnosed with ADHD, especially among adults.
Due to disparities in the treatment and understanding of ADHD between caucasian and non-caucasian populations, many non-caucasian children go undiagnosed and unmedicated. It was found that within the US that there was often a disparity between caucasian and non-caucasian understandings of ADHD. This led to a difference in the classification of the symptoms of ADHD, and therefore, its misdiagnosis. It was also found that it was common in non-caucasian families and teachers to understand the symptoms of ADHD as behavioral issues, rather than mental illness.
Crosscultural differences in diagnosis of ADHD can also be attributed to the long-lasting effects of harmful, racially targeted medical practices. Medical pseudosciences, particularly those that targeted African American populations during the period of slavery in the US, lead to a distrust of medical practices within certain communities. The combination of ADHD symptoms often being regarded as misbehavior rather than as a psychiatric condition, and the use of drugs to regulate ADHD, result in a hesitancy to trust a diagnosis of ADHD. Cases of misdiagnosis in ADHD can also occur due to stereotyping of non-caucasian individuals. Due to ADHD's subjectively determined symptoms, medical professionals may diagnose individuals based on stereotyped behavior or misdiagnose due to differences in symptom presentation between Caucasian and non-Caucasian individuals.
History
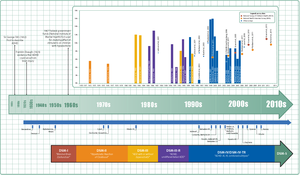
Hyperactivity has long been part of the human condition. Sir Alexander Crichton describes "mental restlessness" in his book An inquiry into the nature and origin of mental derangement written in 1798. He made observations about children showing signs of being inattentive and having the "fidgets". The first clear description of ADHD is credited to George Still in 1902 during a series of lectures he gave to the Royal College of Physicians of London. He noted both nature and nurture could be influencing this disorder.
Alfred Tredgold proposed an association between brain damage and behavioral or learning problems which was able to be validated by the encephalitis lethargica epidemic from 1917 through 1928.
The terminology used to describe the condition has changed over time and has included: minimal brain dysfunction in the DSM-I (1952), hyperkinetic reaction of childhood in the DSM-II (1968), and attention-deficit disorder with or without hyperactivity in the DSM-III (1980). In 1987, this was changed to ADHD in the DSM-III-R, and in 1994 the DSM-IV in split the diagnosis into three subtypes: ADHD inattentive type, ADHD hyperactive-impulsive type, and ADHD combined type. These terms were kept in the DSM-5 in 2013 and in the DSM-5-TR in 2022. Prior to the DSM, terms included minimal brain damage in the 1930s.
In 1934, Benzedrine became the first amphetamine medication approved for use in the United States. Methylphenidate was introduced in the 1950s, and enantiopure dextroamphetamine in the 1970s. The use of stimulants to treat ADHD was first described in 1937. Charles Bradley gave the children with behavioral disorders Benzedrine and found it improved academic performance and behavior.
Once neuroimaging studies were possible, studies conducted in the 1990s provided support for the pre-existing theory that neurological differences - particularly in the frontal lobes - were involved in ADHD. During this same period, a genetic component was identified and ADHD was acknowledged to be a persistent, long-term disorder which lasted from childhood into adulthood.
ADHD was split into the current three sub-types because of a field trial completed by Lahey and colleagues.
Controversy
Main article: Attention deficit hyperactivity disorder controversiesADHD, its diagnosis, and its treatment have been controversial since the 1970s. The controversies involve clinicians, teachers, policymakers, parents, and the media. Positions range from the view that ADHD is within the normal range of behavior to the hypothesis that ADHD is a genetic condition. Other areas of controversy include the use of stimulant medications in children, the method of diagnosis, and the possibility of overdiagnosis. In 2009, the National Institute for Health and Care Excellence, while acknowledging the controversy, states that the current treatments and methods of diagnosis are based on the dominant view of the academic literature. In 2014, Keith Conners, one of the early advocates for recognition of the disorder, spoke out against overdiagnosis in a The New York Times article. In contrast, a 2014 peer-reviewed medical literature review indicated that ADHD is underdiagnosed in adults.
With widely differing rates of diagnosis across countries, states within countries, races, and ethnicities, some suspect factors other than the presence of the symptoms of ADHD are playing a role in diagnosis, such as cultural norms. Some sociologists consider ADHD to be an example of the medicalization of deviant behavior, that is, the turning of the previously non-medical issue of school performance into a medical one. Most healthcare providers accept ADHD as a genuine disorder, at least in the small number of people with severe symptoms. Among healthcare providers the debate mainly centers on diagnosis and treatment in the much greater number of people with mild symptoms.
The nature and range of desirable endpoints of ADHD treatment vary among diagnostic standards for ADHD. In most studies, the efficacy of treatment is determined by reductions in ADHD symptoms. However, some studies have included subjective ratings from teachers and parents as part of their assessment of ADHD treatment efficacies. By contrast, the subjective ratings of children undergoing ADHD treatment are seldom included in studies evaluating the efficacy of ADHD treatments.
There have been notable differences in the diagnosis patterns of birthdays in school-age children. Those born relatively younger to the school starting age than others in a classroom environment are shown to be more likely diagnosed with ADHD. Boys who were born in December in which the school age cut-off was December 31 were shown to be 30% more likely to be diagnosed and 41% to be treated than others born in January. Girls born in December had a diagnosis percentage of 70% and 77% treatment more than ones born the following month. Children who were born at the last 3 days of a calendar year were reported to have significantly higher levels of diagnosis and treatment for ADHD than children born at the first 3 days of a calendar year. The studies suggest that ADHD diagnosis is prone to subjective analysis.
Research directions
Possible positive traits
Possible positive traits of ADHD are a new avenue of research, and therefore limited.
A 2020 review found that creativity may be associated with ADHD symptoms, particularly divergent thinking and quantity of creative achievements, but not with the disorder of ADHD itself – i.e. it has not been found to be increased in people diagnosed with the disorder, only in people with subclinical symptoms or those that possess traits associated with the disorder. Divergent thinking is the ability to produce creative solutions which differ significantly from each other and consider the issue from multiple perspectives. Those with ADHD symptoms could be advantaged in this form of creativity as they tend to have diffuse attention, allowing rapid switching between aspects of the task under consideration; flexible associative memory, allowing them to remember and use more distantly-related ideas which is associated with creativity; and impulsivity, which causes people with ADHD symptoms to consider ideas which others may not have. However, people with ADHD may struggle with convergent thinking, which is a cognitive process through which a set of obviously relevant knowledge is utilized in a focused effort to arrive at a single perceived best solution to a problem.
A 2020 article suggested that historical documentation supported Leonardo da Vinci’s difficulties with procrastination and time management as characteristic of ADHD and that he was constantly on the go, but often jumping from task to task.
Possible biomarkers for diagnosis
Reviews of ADHD biomarkers have noted that platelet monoamine oxidase expression, urinary norepinephrine, urinary MHPG, and urinary phenethylamine levels consistently differ between ADHD individuals and non-ADHD controls. These measurements could potentially serve as diagnostic biomarkers for ADHD, but more research is needed to establish their diagnostic utility. Urinary and blood plasma phenethylamine concentrations are lower in ADHD individuals relative to controls and the two most commonly prescribed drugs for ADHD, amphetamine and methylphenidate, increase phenethylamine biosynthesis in treatment-responsive individuals with ADHD. Lower urinary phenethylamine concentrations are also associated with symptoms of inattentiveness in ADHD individuals.
See also
References
- https://www.forhims.com/blog/adhd-vs-depression
- https://www.heysigmund.com/anxiety-and-adhd/
- ^ Institute for Health Metrics and Evaluation (17 October 2020). "Global Burden of Disease Study 2019: Attention-deficit/hyperactivity disorder—Level 3 cause" (PDF). The Lancet. 396 (10258). Table 1. Archived (PDF) from the original on 7 January 2021. Retrieved 7 January 2021.. Both DSM-IV-TR and ICD-10 criteria were used.
- ^ Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington: American Psychiatric Publishing. 2013. pp. 59–65. ISBN 978-0-89042-555-8.
- ^ Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). Washington, D.C.: American Psychiatric Publishing. February 2022. ISBN 978-0-89042-575-6. OCLC 1288423302.
- ^ "6A05 Attention deficit hyperactivity disorder". International Classification of Diseases 11th Revision. February 2022 . Archived from the original on 1 August 2018. Retrieved 8 May 2022.
- ^ Foreman DM (February 2006). "Attention deficit hyperactivity disorder: legal and ethical aspects". Archives of Disease in Childhood. 91 (2): 192–194. doi:10.1136/adc.2004.064576. PMC 2082674. PMID 16428370.
- ^ Brown TE (October 2008). "ADD/ADHD and Impaired Executive Function in Clinical Practice". Current Psychiatry Reports. 10 (5): 407–411. doi:10.1007/s11920-008-0065-7. PMID 18803914. S2CID 146463279.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 148, 154–157. ISBN 978-0-07-148127-4.
DA has multiple actions in the prefrontal cortex. It promotes the 'cognitive control' of behavior: the selection and successful monitoring of behavior to facilitate attainment of chosen goals. Aspects of cognitive control in which DA plays a role include working memory, the ability to hold information 'on line' in order to guide actions, suppression of prepotent behaviors that compete with goal-directed actions, and control of attention and thus the ability to overcome distractions. Cognitive control is impaired in several disorders, including attention deficit hyperactivity disorder. ... Noradrenergic projections from the LC thus interact with dopaminergic projections from the VTA to regulate cognitive control. ... it has not been shown that 5HT makes a therapeutic contribution to treatment of ADHD.
- ^ Diamond A (2013). "Executive functions". Annual Review of Psychology. 64: 135–168. doi:10.1146/annurev-psych-113011-143750. PMC 4084861. PMID 23020641.
EFs and prefrontal cortex are the first to suffer, and suffer disproportionately, if something is not right in your life. They suffer first, and most, if you are stressed (Arnsten 1998, Liston et al. 2009, Oaten & Cheng 2005), sad (Hirt et al. 2008, von Hecker & Meiser 2005), lonely (Baumeister et al. 2002, Cacioppo & Patrick 2008, Campbell et al. 2006, Tun et al. 2012), sleep deprived (Barnes et al. 2012, Huang et al. 2007), or not physically fit (Best 2010, Chaddock et al. 2011, Hillman et al. 2008). Any of these can cause you to appear to have a disorder of EFs, such as ADHD, when you do not.
- ^ Retz W, Stieglitz RD, Corbisiero S, Retz-Junginger P, Rösler M (October 2012). "Emotional dysregulation in adult ADHD: What is the empirical evidence?". Expert Review of Neurotherapeutics. 12 (10): 1241–1251. doi:10.1586/ern.12.109. PMID 23082740. S2CID 207221320.
- Faraone SV, Rostain AL, Blader J, Busch B, Childress AC, Connor DF, Newcorn JH (February 2019). "Practitioner Review: Emotional dysregulation in attention-deficit/hyperactivity disorder - implications for clinical recognition and intervention". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 60 (2): 133–150. doi:10.1111/jcpp.12899. PMID 29624671.
- Shaw P, Stringaris A, Nigg J, Leibenluft E (March 2014). "Emotion dysregulation in attention deficit hyperactivity disorder". The American Journal of Psychiatry. 171 (3): 276–293. doi:10.1176/appi.ajp.2013.13070966. PMC 4282137. PMID 24480998.
- ^ "Attention Deficit Hyperactivity Disorder (Easy-to-Read)". National Institute of Mental Health. 2013. Archived from the original on 14 April 2016. Retrieved 17 April 2016.
- Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, et al. (October 2018). "Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan". European Neuropsychopharmacology. 28 (10): 1059–1088. doi:10.1016/j.euroneuro.2018.08.001. PMC 6379245. PMID 30195575.
- Faraone SV, Larsson H (April 2019). "Genetics of attention deficit hyperactivity disorder". Molecular Psychiatry. 24 (4): 562–575. doi:10.1038/s41380-018-0070-0. PMC 6477889. PMID 29892054. S2CID 47016805.
- ^ Faraone SV (2011). "Ch. 25: Epidemiology of Attention Deficit Hyperactivity Disorder". In Tsuang MT, Tohen M, Jones P (eds.). Textbook of Psychiatric Epidemiology (3rd ed.). John Wiley & Sons. p. 450. ISBN 9780470977408. Archived from the original on 22 December 2020. Retrieved 1 February 2016.
- Young S, Adamo N, Ásgeirsdóttir BB, Branney P, Beckett M, Colley W, et al. (August 2020). "Females with ADHD: An expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/ hyperactivity disorder in girls and women". BMC Psychiatry. 20 (1): 404. doi:10.1186/s12888-020-02707-9. PMC 7422602. PMID 32787804.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Crawford N (February 2003). "ADHD: a women's issue". Monitor on Psychology. 34 (2): 28. Archived from the original on 9 April 2017.
- ^ Emond V, Joyal C, Poissant H (April 2009). "" [Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)]. L'Encephale (in French). 35 (2): 107–114. doi:10.1016/j.encep.2008.01.005. PMID 19393378.
- ^ Singh I (December 2008). "Beyond polemics: science and ethics of ADHD". Nature Reviews. Neuroscience. 9 (12): 957–964. doi:10.1038/nrn2514. PMID 19020513. S2CID 205504587.
- ^ Song P, Zha M, Yang Q, Zhang Y, Li X, Rudan I (February 2021). "The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis". Journal of Global Health. 11. International Global Health Society: 04009. doi:10.7189/jogh.11.04009. eISSN 2047-2986. OCLC 751737736. PMC 7916320. PMID 33692893.
- ^ Ginsberg Y, Quintero J, Anand E, Casillas M, Upadhyaya HP (2014). "Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature". The Primary Care Companion for CNS Disorders. 16 (3). doi:10.4088/PCC.13r01600. PMC 4195639. PMID 25317367.
Reports indicate that ADHD affects 2.5%–5% of adults in the general population, compared with 5%–7% of children. ... However, fewer than 20% of adults with ADHD are currently diagnosed and/or treated by psychiatrists.
- ^ Coghill DR, Banaschewski T, Soutullo C, Cottingham MG, Zuddas A (November 2017). "Systematic review of quality of life and functional outcomes in randomized placebo-controlled studies of medications for attention-deficit/hyperactivity disorder". European Child & Adolescent Psychiatry. 26 (11): 1283–1307. doi:10.1007/s00787-017-0986-y. PMC 5656703. PMID 28429134.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- "Attention Deficit Hyperactivity Disorder". National Institute of Mental Health. March 2016. Archived from the original on 23 July 2016. Retrieved 5 March 2016.
- ^ National Institute for Health and Care Excellence (2019). Attention deficit hyperactivity disorder: diagnosis and management. NICE Guideline, No. 87. London: National Guideline Centre (UK). ISBN 978-1-4731-2830-9. OCLC 1126668845. Archived from the original on 12 January 2021. Retrieved 9 January 2021.
- ^ "Canadian ADHD Practice Guidelines" (PDF). Canadian ADHD Resource Alliance. Archived (PDF) from the original on 21 January 2021. Retrieved 4 February 2011.
- "Attention-Deficit / Hyperactivity Disorder (ADHD): Recommendations". Centers for Disease Control and Prevention. 24 June 2015. Archived from the original on 7 July 2015. Retrieved 13 July 2015.
- ^ Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. (October 2019). "Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents". Pediatrics. 144 (4): e20192528. doi:10.1542/peds.2019-2528. PMC 7067282. PMID 31570648.
- ^ Wigal SB (2009). "Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults". CNS Drugs. 23 (Suppl 1): 21–31. doi:10.2165/00023210-200923000-00004. PMID 19621975. S2CID 11340058.
- ^ National Collaborating Centre for Mental Health (2009). Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. NICE Clinical Guidelines. Vol. 72. Leicester: British Psychological Society. ISBN 978-1-85433-471-8. Archived from the original on 13 January 2016 – via NCBI Bookshelf.
- Arnold LE, Hodgkins P, Caci H, Kahle J, Young S (February 2015). "Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review". PLOS ONE. 10 (2): e0116407. Bibcode:2015PLoSO..1016407A. doi:10.1371/journal.pone.0116407. PMC 4340791. PMID 25714373.
- ^ Parker J, Wales G, Chalhoub N, Harpin V (September 2013). "The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials". Psychology Research and Behavior Management. 6: 87–99. doi:10.2147/PRBM.S49114. PMC 3785407. PMID 24082796.
Results suggest there is moderate-to-high-level evidence that combined pharmacological and behavioral interventions, and pharmacological interventions alone can be effective in managing the core ADHD symptoms and academic performance at 14 months. However, the effect size may decrease beyond this period. ... Only one paper examining outcomes beyond 36 months met the review criteria. ... There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mayes R, Bagwell C, Erkulwater J (2008). "ADHD and the rise in stimulant use among children". Harvard Review of Psychiatry. 16 (3): 151–166. doi:10.1080/10673220802167782. PMID 18569037. S2CID 18481191.
- Silver LB (2004). Attention-deficit/hyperactivity disorder (3rd ed.). American Psychiatric Publishing. pp. 4–7. ISBN 978-1-58562-131-6.
- Schonwald A, Lechner E (April 2006). "Attention deficit/hyperactivity disorder: complexities and controversies". Current Opinion in Pediatrics. 18 (2): 189–195. doi:10.1097/01.mop.0000193302.70882.70. PMID 16601502. S2CID 27286123.
- ^ Dobie C (2012). "Diagnosis and management of attention deficit hyperactivity disorder in primary care for school-age children and adolescents". p. 79. Archived from the original on 1 March 2013. Retrieved 10 October 2012.
- ^ CDC (6 January 2016). "Facts About ADHD". Centers for Disease Control and Prevention. Archived from the original on 22 March 2016. Retrieved 20 March 2016.
- ^ Ramsay JR (2007). Cognitive behavioral therapy for adult ADHD. Routledge. pp. 4, 25–26. ISBN 978-0-415-95501-0.
- Gershon J (January 2002). "A meta-analytic review of gender differences in ADHD". Journal of Attention Disorders. 5 (3): 143–154. doi:10.1177/108705470200500302. PMID 11911007. S2CID 8076914.
- ^ Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, et al. (September 2010). "European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD". BMC Psychiatry. 10 (67): 67. doi:10.1186/1471-244X-10-67. PMC 2942810. PMID 20815868.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Coleman WL (August 2008). "Social competence and friendship formation in adolescents with attention-deficit/hyperactivity disorder". Adolescent Medicine. 19 (2): 278–99, x. PMID 18822833.
- "ADHD Anger Management Directory". Webmd.com. Archived from the original on 5 November 2013. Retrieved 17 January 2014.
- ^ "F90 Hyperkinetic disorders". International Statistical Classification of Diseases and Related Health Problems 10th Revision. World Health Organisation. 2010. Archived from the original on 2 November 2014. Retrieved 2 November 2014.
- Bellani M, Moretti A, Perlini C, Brambilla P (December 2011). "Language disturbances in ADHD". Epidemiology and Psychiatric Sciences. 20 (4): 311–315. doi:10.1017/S2045796011000527. PMID 22201208.
- Racine MB, Majnemer A, Shevell M, Snider L (April 2008). "Handwriting performance in children with attention deficit hyperactivity disorder (ADHD)". Journal of Child Neurology. 23 (4): 399–406. doi:10.1177/0883073807309244. PMID 18401033. S2CID 206546871.
- Peterson RL, Pennington BF (May 2012). "Developmental dyslexia". Lancet. 379 (9830): 1997–2007. doi:10.1016/S0140-6736(12)60198-6. PMC 3465717. PMID 22513218.
- Sexton CC, Gelhorn HL, Bell JA, Classi PM (November 2012). "The co-occurrence of reading disorder and ADHD: epidemiology, treatment, psychosocial impact, and economic burden". Journal of Learning Disabilities. 45 (6): 538–564. doi:10.1177/0022219411407772. PMID 21757683. S2CID 385238.
- Nicolson RI, Fawcett AJ (January 2011). "Dyslexia, dysgraphia, procedural learning and the cerebellum". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 47 (1): 117–127. doi:10.1016/j.cortex.2009.08.016. PMID 19818437. S2CID 32228208.
- "Dyslexia and ADHD".
- ^ Walitza S, Drechsler R, Ball J (August 2012). "" [The school child with ADHD]. Therapeutische Umschau. Revue Therapeutique (in German). 69 (8): 467–473. doi:10.1024/0040-5930/a000316. PMID 22851461.
- Young S, Hollingdale J, Absoud M, Bolton P, Branney P, Colley W, et al. (May 2020). "Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus". BMC Medicine. 18 (1). Springer Science and Business Media LLC: 146. doi:10.1186/s12916-020-01585-y. PMC 7247165. PMID 32448170.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "ADHD Symptoms". nhs.uk. 20 October 2017. Archived from the original on 1 February 2021. Retrieved 15 May 2018.
- ^ Bailey E. "ADHD and Learning Disabilities: How can you help your child cope with ADHD and subsequent Learning Difficulties? There is a way". Remedy Health Media, LLC. Archived from the original on 3 December 2013. Retrieved 15 November 2013.
- Krull KR (5 December 2007). "Evaluation and diagnosis of attention deficit hyperactivity disorder in children". Uptodate. Wolters Kluwer Health. Archived from the original on 5 June 2009. Retrieved 12 September 2008.
- Hofvander B, Ossowski D, Lundström S, Anckarsäter H (2009). "Continuity of aggressive antisocial behavior from childhood to adulthood: The question of phenotype definition". International Journal of Law and Psychiatry. 32 (4): 224–234. doi:10.1016/j.ijlp.2009.04.004. PMID 19428109. Archived from the original on 17 May 2022. Retrieved 22 November 2021.
- Rubia K (June 2011). ""Cool" inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review". Biological Psychiatry. 69 (12): e69–e87. doi:10.1016/j.biopsych.2010.09.023. PMID 21094938. S2CID 14987165.
- ^ Wilens TE, Spencer TJ (September 2010). "Understanding attention-deficit/hyperactivity disorder from childhood to adulthood". Postgraduate Medicine. 122 (5): 97–109. doi:10.3810/pgm.2010.09.2206. PMC 3724232. PMID 20861593.
- Baud P, Perroud N, Aubry JM (June 2011). "". Revue Médicale Suisse (in French). 7 (297): 1219–1222. PMID 21717696.
- Wilens TE, Morrison NR (July 2011). "The intersection of attention-deficit/hyperactivity disorder and substance abuse". Current Opinion in Psychiatry. 24 (4): 280–285. doi:10.1097/YCO.0b013e328345c956. PMC 3435098. PMID 21483267.
- Corkum P, Davidson F, Macpherson M (June 2011). "A framework for the assessment and treatment of sleep problems in children with attention-deficit/hyperactivity disorder". Pediatric Clinics of North America. 58 (3): 667–683. doi:10.1016/j.pcl.2011.03.004. PMID 21600348.
- Tsai MH, Huang YS (May 2010). "Attention-deficit/hyperactivity disorder and sleep disorders in children". The Medical Clinics of North America. 94 (3): 615–632. doi:10.1016/j.mcna.2010.03.008. PMID 20451036.
- Bendz LM, Scates AC (January 2010). "Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder". The Annals of Pharmacotherapy. 44 (1): 185–191. doi:10.1345/aph.1M365. PMID 20028959. S2CID 207263711.
- Merino-Andreu M (March 2011). "" [Attention deficit hyperactivity disorder and restless legs syndrome in children]. Revista de Neurología (in Spanish). 52 (Suppl 1): S85–S95. doi:10.33588/rn.52S01.2011037. PMID 21365608.
- Picchietti MA, Picchietti DL (August 2010). "Advances in pediatric restless legs syndrome: Iron, genetics, diagnosis and treatment". Sleep Medicine. 11 (7): 643–651. doi:10.1016/j.sleep.2009.11.014. PMID 20620105.
- Karroum E, Konofal E, Arnulf I (2008). "". Revue Neurologique (in French). 164 (8–9): 701–721. doi:10.1016/j.neurol.2008.06.006. PMID 18656214.
- Wajszilber D, Santiseban JA, Gruber R (December 2018). "Sleep disorders in patients with ADHD: impact and management challenges". Nature and Science of Sleep. 10: 453–480. doi:10.2147/NSS.S163074. PMC 6299464. PMID 30588139.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Long Y, Pan N, Ji S, Qin K, Chen Y, Zhang X, et al. (September 2022). "Distinct brain structural abnormalities in attention-deficit/hyperactivity disorder and substance use disorders: A comparative meta-analysis". Translational Psychiatry. 12 (1): 368. doi:10.1038/s41398-022-02130-6. PMC 9448791. PMID 36068207.
- ^ National Collaborating Centre for Mental Health (2009). "Attention Deficit Hyperactivity Disorder". Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. NICE Clinical Guidelines. Vol. 72. Leicester: British Psychological Society. pp. 18–26, 38. ISBN 978-1-85433-471-8. Archived from the original on 13 January 2016 – via NCBI Bookshelf.
- Storebø OJ, Rasmussen PD, Simonsen E (February 2016). "Association Between Insecure Attachment and ADHD: Environmental Mediating Factors" (PDF). Journal of Attention Disorders. 20 (2): 187–196. doi:10.1177/1087054713501079. PMID 24062279. S2CID 23564305. Archived (PDF) from the original on 9 December 2021. Retrieved 22 November 2021.
- Barkley RA (January 2014). "Sluggish cognitive tempo (concentration deficit disorder?): current status, future directions, and a plea to change the name" (PDF). Journal of Abnormal Child Psychology. 42 (1): 117–125. doi:10.1007/s10802-013-9824-y. PMID 24234590. S2CID 8287560. Archived (PDF) from the original on 9 August 2017.
- ^ Nazar BP, Bernardes C, Peachey G, Sergeant J, Mattos P, Treasure J (December 2016). "The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis". The International Journal of Eating Disorders. 49 (12): 1045–1057. doi:10.1002/eat.22643. PMID 27859581. S2CID 38002526.
- ^ Chung W, Jiang SF, Paksarian D, Nikolaidis A, Castellanos FX, Merikangas KR, Milham MP (November 2019). "Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder Among Adults and Children of Different Racial and Ethnic Groups". JAMA Network Open. 2 (11): e1914344. doi:10.1001/jamanetworkopen.2019.14344. PMC 6826640. PMID 31675080.
- Bartoli F, Callovini T, Cavaleri D, Cioni RM, Bachi B, Calabrese A, et al. (July 2022). "Clinical correlates of comorbid attention deficit hyperactivity disorder in adults suffering from bipolar disorder: A meta-analysis". The Australian and New Zealand Journal of Psychiatry: 48674221106669. doi:10.1177/00048674221106669. PMID 35786010. S2CID 250253609.
- ^ Ford JD, Connor DF (1 June 2009). "ADHD and post-traumatic stress disorder". Current Attention Disorders Reports. 1 (2): 60–66. doi:10.1007/s12618-009-0009-0. ISSN 1943-457X. S2CID 145508751.
- Schneider M, VanOrmer J, Zlomke K (2019). "Adverse Childhood Experiences and Family Resilience Among Children with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder". Journal of Developmental and Behavioral Pediatrics. 40 (8): 573–580. doi:10.1097/DBP.0000000000000703. PMID 31335581. S2CID 198193637.
- Moon DS, Bong SJ, Kim BN, Kang NR (January 2021). "Association between Maternal Adverse Childhood Experiences and Attention-Deficit/Hyperactivity Disorder in the Offspring: The Mediating Role of Antepartum Health Risks". Soa--Ch'ongsonyon Chongsin Uihak = Journal of Child & Adolescent Psychiatry. 32 (1): 28–34. doi:10.5765/jkacap.200041. PMC 7788667. PMID 33424239.
- ^ Szymanski K, Sapanski L, Conway F (1 January 2011). "Trauma and ADHD – Association or Diagnostic Confusion? A Clinical Perspective". Journal of Infant, Child, and Adolescent Psychotherapy. 10 (1). Philadelphia PA: Taylor & Francis Group: 51–59. doi:10.1080/15289168.2011.575704. eISSN 1940-9214. ISSN 1528-9168. S2CID 144348893.
- Harrington KM, Miller MW, Wolf EJ, Reardon AF, Ryabchenko KA, Ofrat S (August 2012). "Attention-deficit/hyperactivity disorder comorbidity in a sample of veterans with posttraumatic stress disorder". Comprehensive Psychiatry. 53 (6): 679–690. doi:10.1016/j.comppsych.2011.12.001. PMC 6519447. PMID 22305866.
- Zhang N, Gao M, Yu J, Zhang Q, Wang W, Zhou C, et al. (October 2022). "Understanding the association between adverse childhood experiences and subsequent attention deficit hyperactivity disorder: A systematic review and meta-analysis of observational studies". Brain and Behavior. 12 (10): e32748. doi:10.1002/brb3.2748. PMC 9575611. PMID 36068993.
- Nguyen MN, Watanabe-Galloway S, Hill JL, Siahpush M, Tibbits MK, Wichman C (June 2019). "Ecological model of school engagement and attention-deficit/hyperactivity disorder in school-aged children". European Child & Adolescent Psychiatry. 28 (6): 795–805. doi:10.1007/s00787-018-1248-3. PMID 30390147. S2CID 53263217.
- Miodus S, Allwood MA, Amoh N (5 January 2021). "Childhood ADHD Symptoms in Relation to Trauma Exposure and PTSD Symptoms Among College Students: Attending to and Accommodating Trauma". Journal of Emotional and Behavioral Disorders. 29 (3): 187–196. doi:10.1177/1063426620982624. ISSN 1063-4266. S2CID 234159064. S2CID 234159064.
- Williams AE, Giust JM, Kronenberger WG, Dunn DW (2016). "Epilepsy and attention-deficit hyperactivity disorder: links, risks, and challenges". Neuropsychiatric Disease and Treatment. 12: 287–296. doi:10.2147/NDT.S81549. PMC 4755462. PMID 26929624.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Silva RR, Munoz DM, Alpert M (March 1996). "Carbamazepine use in children and adolescents with features of attention-deficit hyperactivity disorder: a meta-analysis". Journal of the American Academy of Child and Adolescent Psychiatry. 35 (3): 352–358. doi:10.1097/00004583-199603000-00017. PMID 8714324.
- ^ Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J (February 2018). "Adult ADHD and Comorbid Somatic Disease: A Systematic Literature Review". Journal of Attention Disorders (Systematic Review). 22 (3): 203–228. doi:10.1177/1087054716669589. PMC 5987989. PMID 27664125.
- Gaur S (May 2022). "The Association between ADHD and Celiac Disease in Children". Children. 9 (6). MDPI: 781. doi:10.3390/children9060781. PMC 9221618. PMID 35740718.
- Hsu TW, Chen MH, Chu CS, Tsai SJ, Bai YM, Su TP, et al. (May 2022). "Attention deficit hyperactivity disorder and risk of migraine: A nationwide longitudinal study". Headache. 62 (5): 634–641. doi:10.1111/head.14306. PMID 35524451. S2CID 248553863.
- ^ Salem H, Vivas D, Cao F, Kazimi IF, Teixeira AL, Zeni CP (March 2018). "ADHD is associated with migraine: a systematic review and meta-analysis". European Child & Adolescent Psychiatry. 27 (3). Springer Science and Business Media LLC: 267–277. doi:10.1007/s00787-017-1045-4. PMID 28905127. S2CID 3949012.
- ^ Pan PY, Jonsson U, Şahpazoğlu Çakmak SS, Häge A, Hohmann S, Nobel Norrman H, et al. (January 2022). "Headache in ADHD as comorbidity and a side effect of medications: a systematic review and meta-analysis". Psychological Medicine. 52 (1). Cambridge University Press: 14–25. doi:10.1017/s0033291721004141. PMC 8711104. PMID 34635194.
- Cannon Homaei S, Barone H, Kleppe R, Betari N, Reif A, Haavik J (November 2021). "ADHD symptoms in neurometabolic diseases: Underlying mechanisms and clinical implications". Neuroscience and Biobehavioral Reviews. 132: 838–856. doi:10.1016/j.neubiorev.2021.11.012. ISSN 0149-7634. PMID 34774900. S2CID 243983688.
- Balazs J, Kereszteny A (March 2017). "Attention-deficit/hyperactivity disorder and suicide: A systematic review". World Journal of Psychiatry. 7 (1): 44–59. doi:10.5498/wjp.v7.i1.44. PMC 5371172. PMID 28401048.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Garas P, Balazs J (21 December 2020). "Long-Term Suicide Risk of Children and Adolescents With Attention Deficit and Hyperactivity Disorder-A Systematic Review". Frontiers in Psychiatry. 11: 557909. doi:10.3389/fpsyt.2020.557909. PMC 7779592. PMID 33408650. 557909.
- ^ Septier M, Stordeur C, Zhang J, Delorme R, Cortese S (August 2019). "Association between suicidal spectrum behaviors and Attention-Deficit/Hyperactivity Disorder: A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 103: 109–118. doi:10.1016/j.neubiorev.2019.05.022. PMID 31129238. S2CID 162184004. Archived from the original on 4 November 2021. Retrieved 7 December 2021.
- Beauchaine TP, Ben-David I, Bos M (September 2020). "ADHD, financial distress, and suicide in adulthood: A population study". Science Advances. 6 (40): eaba1551. Bibcode:2020SciA....6.1551B. doi:10.1126/sciadv.aba1551. PMC 7527218. PMID 32998893. eaba1551.
- ^ Frazier TW, Demaree HA, Youngstrom EA (July 2004). "Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder". Neuropsychology. 18 (3): 543–555. doi:10.1037/0894-4105.18.3.543. PMID 15291732. S2CID 17628705.
- Mackenzie GB, Wonders E (2016). "Rethinking Intelligence Quotient Exclusion Criteria Practices in the Study of Attention Deficit Hyperactivity Disorder". Frontiers in Psychology. 7: 794. doi:10.3389/fpsyg.2016.00794. PMC 4886698. PMID 27303350.
- Rommelse N, van der Kruijs M, Damhuis J, Hoek I, Smeets S, Antshel KM, et al. (December 2016). "An evidenced-based perspective on the validity of attention-deficit/hyperactivity disorder in the context of high intelligence". Neuroscience and Biobehavioral Reviews. 71: 21–47. doi:10.1016/j.neubiorev.2016.08.032. PMID 27590827. S2CID 6698847.
- Bridgett DJ, Walker ME (March 2006). "Intellectual functioning in adults with ADHD: a meta-analytic examination of full scale IQ differences between adults with and without ADHD". Psychological Assessment. 18 (1): 1–14. doi:10.1037/1040-3590.18.1.1. PMID 16594807.
- ^ Millichap JG (2010). "Chapter 2: Causative Factors". Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, NY: Springer Science. p. 26. doi:10.1007/978-1-4419-1397-5. ISBN 978-1-4419-1396-8. LCCN 2009938108. Archived from the original on 22 December 2020. Retrieved 1 February 2016.
- ^ Thapar A, Cooper M, Eyre O, Langley K (January 2013). "What have we learnt about the causes of ADHD?". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 54 (1): 3–16. doi:10.1111/j.1469-7610.2012.02611.x. PMC 3572580. PMID 22963644.
- Scerif G, Baker K (March 2015). "Annual research review: Rare genotypes and childhood psychopathology--uncovering diverse developmental mechanisms of ADHD risk". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 56 (3): 251–273. doi:10.1111/jcpp.12374. PMID 25494546.
- ^ Faraone SV, Larsson H (April 2019). "Genetics of attention deficit hyperactivity disorder". Molecular Psychiatry. 24 (4). Springer Science and Business Media LLC: 562–575. doi:10.1038/s41380-018-0070-0. PMC 6477889. PMID 29892054.
- Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. (September 2021). "The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder". Neuroscience and Biobehavioral Reviews. 128. Elsevier BV: 789–818. doi:10.1016/j.neubiorev.2021.01.022. PMC 8328933. PMID 33549739.
- Nolen-Hoeksema S (2013). Abnormal Psychology (Sixth ed.). p. 267. ISBN 978-0-07-803538-8.
- Hinshaw SP (May 2018). "Attention Deficit Hyperactivity Disorder (ADHD): Controversy, Developmental Mechanisms, and Multiple Levels of Analysis". Annual Review of Clinical Psychology. 14 (1): 291–316. doi:10.1146/annurev-clinpsy-050817-084917. PMID 29220204.
- ^ Gizer IR, Ficks C, Waldman ID (July 2009). "Candidate gene studies of ADHD: a meta-analytic review". Human Genetics. 126 (1): 51–90. doi:10.1007/s00439-009-0694-x. PMID 19506906. S2CID 166017.
- ^ Kebir O, Joober R (December 2011). "Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: a review of genetic association studies". European Archives of Psychiatry and Clinical Neuroscience. 261 (8): 583–594. doi:10.1007/s00406-011-0207-5. PMID 21409419. S2CID 21383749.
- ^ Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Reviews on Recent Clinical Trials. 2 (1): 3–19. CiteSeerX 10.1.1.329.563. doi:10.2174/157488707779318107. PMID 18473983.
Although there is little direct evidence, changes in trace amines, in particular PE, have been identified as a possible factor for the onset of attention deficit/hyperactivity disorder (ADHD). … Further, amphetamines, which have clinical utility in ADHD, are good ligands at trace amine receptors. Of possible relevance in this aspect is modafanil, which has shown beneficial effects in ADHD patients and has been reported to enhance the activity of PE at TAAR1. Conversely, methylphenidate, …showed poor efficacy at the TAAR1 receptor. In this respect it is worth noting that the enhancement of functioning at TAAR1 seen with modafanil was not a result of a direct interaction with TAAR1.
- Sotnikova TD, Caron MG, Gainetdinov RR (August 2009). "Trace amine-associated receptors as emerging therapeutic targets". Molecular Pharmacology. 76 (2): 229–235. doi:10.1124/mol.109.055970. PMC 2713119. PMID 19389919.
- Arcos-Burgos M, Muenke M (November 2010). "Toward a better understanding of ADHD: LPHN3 gene variants and the susceptibility to develop ADHD". Attention Deficit and Hyperactivity Disorders. 2 (3): 139–147. doi:10.1007/s12402-010-0030-2. PMC 3280610. PMID 21432600.
- Nikolaidis A, Gray JR (June 2010). "ADHD and the DRD4 exon III 7-repeat polymorphism: an international meta-analysis". Social Cognitive and Affective Neuroscience. 5 (2–3): 188–193. doi:10.1093/scan/nsp049. PMC 2894686. PMID 20019071.
- ^ Grimm O, Kranz TM, Reif A (February 2020). "Genetics of ADHD: What Should the Clinician Know?". Current Psychiatry Reports. 22 (4): 18. doi:10.1007/s11920-020-1141-x. PMC 7046577. PMID 32108282.
- ^ Zayats T, Neale BM (12 February 2020). "Recent advances in understanding of attention deficit hyperactivity disorder (ADHD): how genetics are shaping our conceptualization of this disorder". F1000Research. 8: 2060. doi:10.12688/f1000research.18959.2. PMC 6896240. PMID 31824658.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. (March 2013). "Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments". The American Journal of Psychiatry. 170 (3): 275–289. doi:10.1176/appi.ajp.2012.12070991. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183. PMID 23360949. S2CID 434310.
Free fatty acid supplementation and artificial food color exclusions appear to have beneficial effects on ADHD symptoms, although the effect of the former are small and those of the latter may be limited to ADHD patients with food sensitivities...
- CDC (16 March 2016). "Attention-Deficit / Hyperactivity Disorder (ADHD)". Centers for Disease Control and Prevention. Archived from the original on 14 April 2016. Retrieved 17 April 2016.
- Burger PH, Goecke TW, Fasching PA, Moll G, Heinrich H, Beckmann MW, Kornhuber J (September 2011). "Einfluss des mütterlichen Alkoholkonsums während der Schwangerschaft auf die Entwicklung von ADHS beim Kind" [How does maternal alcohol consumption during pregnancy affect the development of attention deficit/hyperactivity syndrome in the child]. Fortschritte der Neurologie-Psychiatrie (Review) (in German). 79 (9): 500–506. doi:10.1055/s-0031-1273360. PMID 21739408.
- Eubig PA, Aguiar A, Schantz SL (December 2010). "Lead and PCBs as risk factors for attention deficit/hyperactivity disorder". Environmental Health Perspectives (Review. Research Support, N.I.H., Extramural. Research Support, U.S. Gov't, Non-P.H.S.). 118 (12): 1654–1667. doi:10.1289/ehp.0901852. PMC 3002184. PMID 20829149.
- de Cock M, Maas YG, van de Bor M (August 2012). "Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review". Acta Paediatrica (Review. Research Support, Non-U.S. Gov't). 101 (8): 811–818. doi:10.1111/j.1651-2227.2012.02693.x. PMID 22458970. S2CID 41748237.
- Abbott LC, Winzer-Serhan UH (April 2012). "Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models". Critical Reviews in Toxicology (Review). 42 (4): 279–303. doi:10.3109/10408444.2012.658506. PMID 22394313. S2CID 38886526.
- Tiesler CM, Heinrich J (October 2014). "Prenatal nicotine exposure and child behavioural problems". European Child & Adolescent Psychiatry. 23 (10): 913–929. doi:10.1007/s00787-014-0615-y. PMC 4186967. PMID 25241028.
- Botting N, Powls A, Cooke RW, Marlow N (November 1997). "Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 38 (8): 931–941. doi:10.1111/j.1469-7610.1997.tb01612.x. PMID 9413793. Archived from the original on 17 May 2022. Retrieved 22 March 2022.
- Thapar A, Cooper M, Jefferies R, Stergiakouli E (March 2012). "What causes attention deficit hyperactivity disorder?". Archives of Disease in Childhood (Review. Research Support, Non-U.S. Gov't). 97 (3): 260–265. doi:10.1136/archdischild-2011-300482. PMC 3927422. PMID 21903599.
- Millichap JG (February 2008). "Etiologic classification of attention-deficit/hyperactivity disorder". Pediatrics (Review). 121 (2): e358–e365. doi:10.1542/peds.2007-1332. PMID 18245408. S2CID 24339363.
- Eme R (April 2012). "ADHD: an integration with pediatric traumatic brain injury". Expert Review of Neurotherapeutics (Review). 12 (4): 475–483. doi:10.1586/ern.12.15. PMID 22449218. S2CID 35718630.
- ^ Mayes R, Bagwell C, Erkulwater JL (2009). Medicating Children: ADHD and Pediatric Mental Health (illustrated ed.). Harvard University Press. pp. 4–24. ISBN 978-0-674-03163-0.
- ^ Millichap JG, Yee MM (February 2012). "The diet factor in attention-deficit/hyperactivity disorder". Pediatrics. 129 (2): 330–337. doi:10.1542/peds.2011-2199. PMID 22232312. S2CID 14925322. Archived from the original on 11 September 2015.
- Tomaska LD, Brooke-Taylor S (2014). "Food Additives – General". In Motarjemi Y, Moy GG, Todd EC (eds.). Encyclopedia of Food Safety. Vol. 3 (1st ed.). Amsterdam: Elsevier/Academic Press. pp. 449–54. ISBN 978-0-12-378613-5. OCLC 865335120.
- "Background Document for the Food Advisory Committee: Certified Color Additives in Food and Possible Association with Attention Deficit Hyperactivity Disorder in Children" (PDF). U.S. Food and Drug Administration. March 2011. Archived (PDF) from the original on 6 November 2015.
- ^ Nigg JT, Holton K (October 2014). "Restriction and elimination diets in ADHD treatment". Child and Adolescent Psychiatric Clinics of North America (Review). 23 (4): 937–953. doi:10.1016/j.chc.2014.05.010. PMC 4322780. PMID 25220094.
an elimination diet produces a small aggregate effect but may have greater benefit among some children. Very few studies enable proper evaluation of the likelihood of response in children with ADHD who are not already preselected based on prior diet response.
- Holland J, Sayal K (November 2019). "Relative age and ADHD symptoms, diagnosis and medication: a systematic review". European Child & Adolescent Psychiatry. 28 (11): 1417–1429. doi:10.1007/s00787-018-1229-6. PMC 6800871. PMID 30293121.
- Parritz R (2013). Disorders of Childhood: Development and Psychopathology. Cengage Learning. pp. 151. ISBN 978-1-285-09606-3.
- Stockman JA (2016). Year Book of Pediatrics 2014 E-Book. Elsevier Health Sciences. p. 163. ISBN 9780323265270. Archived from the original on 26 July 2020. Retrieved 4 June 2020.
- "Mental health of children and adolescents" (PDF). WHO Europe. 15 January 2005. Archived from the original (PDF) on 24 October 2009. Retrieved 13 October 2011.
- ^ Chandler DJ, Waterhouse BD, Gao WJ (May 2014). "New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons". Frontiers in Neural Circuits. 8: 53. doi:10.3389/fncir.2014.00053. PMC 4033238. PMID 24904299.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapters 10 and 13". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 266, 315, 318–323. ISBN 978-0-07-148127-4.
Early results with structural MRI show thinning of the cerebral cortex in ADHD subjects compared with age-matched controls in prefrontal cortex and posterior parietal cortex, areas involved in working memory and attention.
- ^ Castellanos FX, Proal E (January 2012). "Large-scale brain systems in ADHD: beyond the prefrontal-striatal model". Trends in Cognitive Sciences. 16 (1): 17–26. doi:10.1016/j.tics.2011.11.007. PMC 3272832. PMID 22169776.
Recent conceptualizations of ADHD have taken seriously the distributed nature of neuronal processing. Most of the candidate networks have focused on prefrontal-striatal-cerebellar circuits, although other posterior regions are also being proposed.
- ^ Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX (October 2012). "Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies". The American Journal of Psychiatry. 169 (10): 1038–1055. doi:10.1176/appi.ajp.2012.11101521. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183. PMC 3879048. PMID 22983386.
- Krain AL, Castellanos FX (August 2006). "Brain development and ADHD". Clinical Psychology Review. 26 (4): 433–444. doi:10.1016/j.cpr.2006.01.005. PMID 16480802.
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LS, et al. (April 2017). "Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis". The Lancet. Psychiatry. 4 (4): 310–319. doi:10.1016/S2215-0366(17)30049-4. PMC 5933934. PMID 28219628.
- Douglas PK, Gutman B, Anderson A, Larios C, Lawrence KE, Narr K, Sengupta B, Coorray G, Douglas DB, Thompson PM, McGough JJ, Bookheimer SY (February 2018). "Hemispheric brain asymmetry differences in youths with attention-deficit/hyperactivity disorder". NeuroImage: Clinical. 18: 744–752. doi:10.1016/j.nicl.2018.02.020. PMC 5988460. PMID 29876263.
- Colby JB, Rudie JD, Brown JA, Douglas PK, Cohen MS, Shehzad Z (August 2012). "Insights into multimodal imaging classification of ADHD". Frontiers in Systems Neuroscience. 6: 59. doi:10.3389/fnsys.2012.00059. PMC 3419970. PMID 22912605.
- Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U (March 2012). "Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis". The American Journal of Psychiatry. 169 (3): 264–272. doi:10.1176/appi.ajp.2011.11060940. eISSN 1535-7228. hdl:11577/2482784. ISSN 0002-953X. LCCN 22024537. OCLC 1480183. PMID 22294258.
- ^ Bidwell LC, McClernon FJ, Kollins SH (August 2011). "Cognitive enhancers for the treatment of ADHD". Pharmacology, Biochemistry, and Behavior. 99 (2): 262–274. doi:10.1016/j.pbb.2011.05.002. PMC 3353150. PMID 21596055.
- Cortese S (September 2012). "The neurobiology and genetics of Attention-Deficit/Hyperactivity Disorder (ADHD): what every clinician should know". European Journal of Paediatric Neurology. 16 (5): 422–433. doi:10.1016/j.ejpn.2012.01.009. PMID 22306277.
- Lesch KP, Merker S, Reif A, Novak M (June 2013). "Dances with black widow spiders: dysregulation of glutamate signalling enters centre stage in ADHD". European Neuropsychopharmacology. 23 (6): 479–491. doi:10.1016/j.euroneuro.2012.07.013. PMID 22939004. S2CID 14701654.
- Skodzik T, Holling H, Pedersen A (February 2017). "Long-Term Memory Performance in Adult ADHD". Journal of Attention Disorders. 21 (4): 267–283. doi:10.1177/1087054713510561. PMID 24232170. S2CID 27070077.
- ^ Modesto-Lowe V, Chaplin M, Soovajian V, Meyer A (July 2013). "Are motivation deficits underestimated in patients with ADHD? A review of the literature". Postgraduate Medicine. 125 (4): 47–52. doi:10.3810/pgm.2013.07.2677. PMID 23933893. S2CID 24817804.
Behavioral studies show altered processing of reinforcement and incentives in children with ADHD. These children respond more impulsively to rewards and choose small, immediate rewards over larger, delayed incentives. Interestingly, a high intensity of reinforcement is effective in improving task performance in children with ADHD. Pharmacotherapy may also improve task persistence in these children. ... Previous studies suggest that a clinical approach using interventions to improve motivational processes in patients with ADHD may improve outcomes as children with ADHD transition into adolescence and adulthood.
- B. Langguth, R. Bär, N. Wodarz, M. Wittmann, R. Laufkötter: Paradoxical reaction in ADHD. In: Deutsches Ärzteblatt international. Band 108, Nummer 31–32, August 2011, S. 541; author reply 541–541; author reply 542, (in German).doi:10.3238/arztebl.2011.0541a, PMID 21886668, PMC 3163785.
- Rainer Laufkötter, Berthold Langguth, Monika Johann, Peter Eichhammer, Göran Hajak: ADHS des Erwachsenenalters und Komorbiditäten. In: psychoneuro. 31, 2005, S. 563, (in German).doi:10.1055/s-2005-923370.
- Dulcan MK, Lake MB (2011). "Axis I Disorders Usually First Diagnosed in Infancy, Childhood or Adolescence: Attention-Deficit and Disruptive Behavior Disorders". Concise Guide to Child and Adolescent Psychiatry (4th illustrated ed.). American Psychiatric Publishing. pp. 34. ISBN 978-1-58562-416-4 – via Google Books.
- ^ National Collaborating Centre for Mental Health (2009). "Diagnosis". Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. NICE Clinical Guidelines. Vol. 72. Leicester: British Psychological Society. pp. 116–7, 119. ISBN 978-1-85433-471-8. Archived from the original on 13 January 2016 – via NCBI Bookshelf.
- "MerckMedicus Modules: ADHD –Pathophysiology". August 2002. Archived from the original on 1 May 2010.
- Caroline SC, ed. (2010). Encyclopedia of Cross-Cultural School Psychology. Springer Science & Business Media. p. 133. ISBN 9780387717982. Archived from the original on 22 December 2020. Retrieved 1 February 2016.
- Wiener JM, Dulcan MK (2004). Textbook Of Child and Adolescent Psychiatry (illustrated ed.). American Psychiatric Publishing. ISBN 978-1-58562-057-9. Archived from the original on 6 May 2016. Retrieved 2 November 2014.
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. (November 2011). "ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents". Pediatrics. 128 (5): 1007–1022. doi:10.1542/peds.2011-2654. PMC 4500647. PMID 22003063.
- Smith BJ, Barkley RA, Shapiro CJ (2007). "Attention-Deficit/Hyperactivity Disorder". In Mash EJ, Barkley RA (eds.). Assessment of Childhood Disorders (4th ed.). New York, NY: Guilford Press. pp. 53–131. ISBN 978-1-59385-493-5.
- Al Rahbi HA, Al-Sabri RM, Chitme HR (April 2014). "Interventions by pharmacists in out-patient pharmaceutical care". Saudi Pharmaceutical Journal. 22 (2): 101–106. doi:10.1016/j.jsps.2013.04.001. PMC 3950532. PMID 24648820.
- "Adult ADHD: Diagnosis". CAMH. Archived from the original on 21 June 2021. Retrieved 17 April 2022.
- Berger I (September 2011). "Diagnosis of attention deficit hyperactivity disorder: much ado about something" (PDF). The Israel Medical Association Journal. 13 (9): 571–574. PMID 21991721. Archived (PDF) from the original on 28 July 2020. Retrieved 23 May 2013.
- Steinau S (2013). "Diagnostic Criteria in Attention Deficit Hyperactivity Disorder - Changes in DSM 5". Frontiers in Psychiatry. 4: 49. doi:10.3389/fpsyt.2013.00049. PMC 3667245. PMID 23755024.
- Parens E, Johnston J (January 2009). "Facts, values, and attention-deficit hyperactivity disorder (ADHD): an update on the controversies". Child and Adolescent Psychiatry and Mental Health. 3 (1): 1. doi:10.1186/1753-2000-3-1. PMC 2637252. PMID 19152690.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Szasz T (2001). "Psychiatric Medicine: Disorder". Pharmacracy: medicine and politics in America. Westport, CT: Praeger. pp. 101. ISBN 978-0-275-97196-0 – via Google Books.
Mental diseases are invented and then given a name, for example attention deficit hyperactivity disorder (ADHD).
- Culpepper L, Mattingly G (2010). "Challenges in identifying and managing attention-deficit/hyperactivity disorder in adults in the primary care setting: a review of the literature". Primary Care Companion to the Journal of Clinical Psychiatry. 12 (6): PCC.10r00951. doi:10.4088/PCC.10r00951pur. PMC 3067998. PMID 21494335.
- ^ Gentile JP, Atiq R, Gillig PM (August 2006). "Adult ADHD: Diagnosis, Differential Diagnosis, and Medication Management". Psychiatry. 3 (8): 25–30. PMC 2957278. PMID 20963192.
likelihood that the adult with ADHD has developed coping mechanisms to compensate for his or her impairment
- Mohr-Jensen C, Steinhausen HC (August 2016). "A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations". Clinical Psychology Review. 48: 32–42. doi:10.1016/j.cpr.2016.05.002. PMID 27390061.
- Asherson P, Agnew-Blais J (April 2019). "Annual Research Review: Does late-onset attention-deficit/hyperactivity disorder exist?". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 60 (4): 333–352. doi:10.1111/jcpp.13020. PMID 30843223.
- Consumer Reports; Drug Effectiveness Review Project (March 2012). "Evaluating Prescription Drugs Used to Treat: Attention Deficit Hyperactivity Disorder (ADHD) Comparing Effectiveness, Safety, and Price" (PDF). Best Buy Drugs: 2. Archived (PDF) from the original on 15 November 2012. Retrieved 12 April 2013.
- ^ Ertürk E, Wouters S, Imeraj L, Lampo A (August 2020). "Association of ADHD and Celiac Disease: What Is the Evidence? A Systematic Review of the Literature". Journal of Attention Disorders (Review). 24 (10): 1371–1376. doi:10.1177/1087054715611493. PMID 26825336. S2CID 33989148.
Up till now, there is no conclusive evidence for a relationship between ADHD and CD. Therefore, it is not advised to perform routine screening of CD when assessing ADHD (and vice versa) or to implement GFD as a standard treatment in ADHD. Nevertheless, the possibility of untreated CD predisposing to ADHD-like behavior should be kept in mind. ... It is possible that in untreated patients with CD, neurologic symptoms such as chronic fatigue, inattention, pain, and headache could predispose patients to ADHD-like behavior (mainly symptoms of inattentive type), which may be alleviated after GFD treatment.
- Owens JA (October 2008). "Sleep disorders and attention-deficit/hyperactivity disorder". Current Psychiatry Reports. 10 (5): 439–444. doi:10.1007/s11920-008-0070-x. PMID 18803919. S2CID 23624443.
- Walters AS, Silvestri R, Zucconi M, Chandrashekariah R, Konofal E (December 2008). "Review of the possible relationship and hypothetical links between attention deficit hyperactivity disorder (ADHD) and the simple sleep related movement disorders, parasomnias, hypersomnias, and circadian rhythm disorders". Journal of Clinical Sleep Medicine. 4 (6): 591–600. doi:10.5664/jcsm.27356. PMC 2603539. PMID 19110891.
- ^ Lal C, Strange C, Bachman D (June 2012). "Neurocognitive impairment in obstructive sleep apnea". Chest. 141 (6): 1601–1610. doi:10.1378/chest.11-2214. PMID 22670023.
- Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE (September 2012). "A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment". BMC Medicine. 10: 99. doi:10.1186/1741-7015-10-99. PMC 3520745. PMID 22947230.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Fabiano GA, Pelham WE, Coles EK, Gnagy EM, Chronis-Tuscano A, O'Connor BC (March 2009). "A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder". Clinical Psychology Review. 29 (2): 129–140. doi:10.1016/j.cpr.2008.11.001. PMID 19131150.
there is strong and consistent evidence that behavioral treatments are effective for treating ADHD.
- Kratochvil CJ, Vaughan BS, Barker A, Corr L, Wheeler A, Madaan V (March 2009). "Review of pediatric attention deficit/hyperactivity disorder for the general psychiatrist". The Psychiatric Clinics of North America. 32 (1): 39–56. doi:10.1016/j.psc.2008.10.001. PMID 19248915.
- Lopez PL, Torrente FM, Ciapponi A, Lischinsky AG, Cetkovich-Bakmas M, Rojas JI, et al. (March 2018). "Cognitive-behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults". The Cochrane Database of Systematic Reviews. 2018 (3): CD010840. doi:10.1002/14651858.CD010840.pub2. PMC 6494390. PMID 29566425.
- Evans SW, Owens JS, Bunford N (2014). "Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder". Journal of Clinical Child and Adolescent Psychology. 43 (4): 527–551. doi:10.1080/15374416.2013.850700. PMC 4025987. PMID 24245813.
- Van Doren J, Arns M, Heinrich H, Vollebregt MA, Strehl U, K Loo S (March 2019). "Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis". European Child & Adolescent Psychiatry. 28 (3). Springer Science and Business Media LLC: 293–305. doi:10.1007/s00787-018-1121-4. PMC 6404655. PMID 29445867.
- Enriquez-Geppert S, Smit D, Pimenta MG, Arns M (May 2019). "Neurofeedback as a Treatment Intervention in ADHD: Current Evidence and Practice". Current Psychiatry Reports. 21 (6). Springer Science and Business Media LLC: 46. doi:10.1007/s11920-019-1021-4. PMC 6538574. PMID 31139966.
- Daley D, Van Der Oord S, Ferrin M, Cortese S, Danckaerts M, Doepfner M, et al. (September 2018). "Practitioner Review: Current best practice in the use of parent training and other behavioural interventions in the treatment of children and adolescents with attention deficit hyperactivity disorder". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 59 (9). Wiley: 932–947. doi:10.1111/jcpp.12825. hdl:11343/293788. PMID 29083042. S2CID 31044370. Archived from the original on 25 September 2017. Retrieved 21 November 2018.
- Bjornstad G, Montgomery P (April 2005). Bjornstad GJ (ed.). "Family therapy for attention-deficit disorder or attention-deficit/hyperactivity disorder in children and adolescents". The Cochrane Database of Systematic Reviews (2): CD005042. doi:10.1002/14651858.CD005042.pub2. PMID 15846741. S2CID 27339381.
- Turkington C, Harris J (2009). "Attention deficit hyperactivity disorder (ADHD)". The Encyclopedia of the Brain and Brain Disorders. Infobase Publishing. pp. 47. ISBN 978-1-4381-2703-3 – via Google Books.
- Mikami AY (June 2010). "The importance of friendship for youth with attention-deficit/hyperactivity disorder". Clinical Child and Family Psychology Review. 13 (2): 181–198. doi:10.1007/s10567-010-0067-y. PMC 2921569. PMID 20490677.
- ^ Dodson WW (May 2005). "Pharmacotherapy of adult ADHD". Journal of Clinical Psychology. 61 (5): 589–606. doi:10.1002/jclp.20122. PMID 15723384.
For example, pseudoephedrine and ephedrine ... have no detectable effects on the symptoms of ADHD.
- ^ Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallón S, Alvarez Zallo N, Luis EO, et al. (January 2018). "Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 84: 63–71. doi:10.1016/j.neubiorev.2017.11.007. PMID 29162520.
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, et al. (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". The Journal of Clinical Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- Frodl T, Skokauskas N (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects". Acta Psychiatrica Scandinavica. 125 (2): 114–126. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249. S2CID 25954331.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. (September 2018). "Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis". The Lancet. Psychiatry. 5 (9): 727–738. doi:10.1016/S2215-0366(18)30269-4. PMC 6109107. PMID 30097390.
- Wynchank D, Bijlenga D, Beekman AT, Kooij JJ, Penninx BW (October 2017). "Adult Attention-Deficit/Hyperactivity Disorder (ADHD) and Insomnia: an Update of the Literature". Current Psychiatry Reports. 19 (12). Springer Science and Business Media LLC: 98. doi:10.1007/s11920-017-0860-0. PMID 29086065. S2CID 38064951.
In varying percentages of trial participants, insomnia is a treatment-emergent adverse effect in triple-bead mixed amphetamine salts (40–45%), dasotraline (35–45%), lisdexamfetamine (10–19%), and extended-release methylphenidate (11%).
- Shoptaw SJ, Kao U, Ling W (January 2009). Shoptaw SJ, Ali R (eds.). "Treatment for amphetamine psychosis". The Cochrane Database of Systematic Reviews. 2009 (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMC 7004251. PMID 19160215.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Archived (PDF) from the original on 30 December 2013. Retrieved 30 December 2013.
Treatment-emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual doses. ... In a pooled analysis of multiple short-term, placebo controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
- Mosholder AD, Gelperin K, Hammad TA, Phelan K, Johann-Liang R (February 2009). "Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children". Pediatrics. 123 (2): 611–616. doi:10.1542/peds.2008-0185. PMID 19171629. S2CID 22391693.
- Ashton H, Gallagher P, Moore B (September 2006). "The adult psychiatrist's dilemma: psychostimulant use in attention deficit/hyperactivity disorder". Journal of Psychopharmacology. 20 (5): 602–610. doi:10.1177/0269881106061710. PMID 16478756. S2CID 32073083.
- Castells X, Blanco-Silvente L, Cunill R, et al. (Cochrane Developmental, Psychosocial and Learning Problems Group) (August 2018). "Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults". The Cochrane Database of Systematic Reviews. 2018 (8): CD007813. doi:10.1002/14651858.CD007813.pub3. PMC 6513464. PMID 30091808.
- ^ Kiely B, Adesman A (June 2015). "What we do not know about ADHD… yet". Current Opinion in Pediatrics. 27 (3): 395–404. doi:10.1097/MOP.0000000000000229. PMID 25888152. S2CID 39004402.
In addition, a consensus has not been reached on the optimal diagnostic criteria for ADHD. Moreover, the benefits and long-term effects of medical and complementary therapies for this disorder continue to be debated. These gaps in knowledge hinder the ability of clinicians to effectively recognise and treat ADHD.
- Hazell P (July 2011). "The challenges to demonstrating long-term effects of psychostimulant treatment for attention-deficit/hyperactivity disorder". Current Opinion in Psychiatry. 24 (4): 286–290. doi:10.1097/YCO.0b013e32834742db. PMID 21519262. S2CID 21998152. Archived from the original on 26 July 2020. Retrieved 19 July 2019.
- Kemper AR, Maslow GR, Hill S, Namdari B, Allen LaPointe NM, Goode AP, et al. (2018). "Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents". Comparative Effectiveness Reviews (203). Rockville (MD): Agency for Healthcare Research and Quality (US). PMID 29558081. Archived from the original on 17 May 2022. Retrieved 7 November 2021.
- Kraemer M, Uekermann J, Wiltfang J, Kis B (July 2010). "Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature". Clinical Neuropharmacology. 33 (4): 204–206. doi:10.1097/WNF.0b013e3181e29174. PMID 20571380. S2CID 34956456.
- van de Loo-Neus GH, Rommelse N, Buitelaar JK (August 2011). "To stop or not to stop? How long should medication treatment of attention-deficit hyperactivity disorder be extended?". European Neuropsychopharmacology. 21 (8): 584–599. doi:10.1016/j.euroneuro.2011.03.008. PMID 21530185. S2CID 30068561.
- Ibrahim K, Donyai P (July 2015). "Drug Holidays From ADHD Medication: International Experience Over the Past Four Decades". Journal of Attention Disorders. 19 (7): 551–568. doi:10.1177/1087054714548035. PMID 25253684. S2CID 19949563. Archived (PDF) from the original on 30 June 2016.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 323, 368. ISBN 978-0-07-148127-4.
supervised use of stimulants at therapeutic doses may decrease risk of experimentation with drugs to self-medicate symptoms. Second, untreated ADHD may lead to school failure, peer rejection, and subsequent association with deviant peer groups that encourage drug misuse. ... amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction
- Oregon Health & Science University (2009). Black box warnings of ADHD drugs approved by the US Food and Drug Administration. Portland, Oregon: United States National Library of Medicine. Archived from the original on 8 September 2017. Retrieved 17 January 2014.
- Potter AS, Schaubhut G, Shipman M (December 2014). "Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: rationale and progress to date". CNS Drugs. 28 (12): 1103–1113. doi:10.1007/s40263-014-0208-9. PMC 4487649. PMID 25349138.
- Ioannidis K, Chamberlain SR, Müller U (September 2014). "Ostracising caffeine from the pharmacological arsenal for attention-deficit hyperactivity disorder--was this a correct decision? A literature review". Journal of Psychopharmacology. 28 (9): 830–836. doi:10.1177/0269881114541014. PMID 24989644. S2CID 13465319.
- Turner D (April 2006). "A review of the use of modafinil for attention-deficit hyperactivity disorder". Expert Review of Neurotherapeutics. 6 (4): 455–468. doi:10.1586/14737175.6.4.455. PMID 16623645. S2CID 24293088.
- Huss M, Chen W, Ludolph AG (January 2016). "Guanfacine Extended Release: A New Pharmacological Treatment Option in Europe". Clinical Drug Investigation. 36 (1). Springer Science and Business Media LLC: 1–25. doi:10.1007/s40261-015-0336-0. PMC 4706844. PMID 26585576.
- Childress AC, Sallee FR (March 2012). "Revisiting clonidine: an innovative add-on option for attention-deficit/hyperactivity disorder". Drugs of Today. 48 (3): 207–217. doi:10.1358/dot.2012.48.3.1750904. PMID 22462040.
- ^ McDonagh MS, Peterson K, Thakurta S, Low A (December 2011). Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder (Report). Drug Class Reviews. United States Library of Medicine. PMID 22420008. Archived from the original on 31 August 2016.
- Prasad V, Brogan E, Mulvaney C, Grainge M, Stanton W, Sayal K (April 2013). "How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis". European Child & Adolescent Psychiatry. 22 (4): 203–216. doi:10.1007/s00787-012-0346-x. PMID 23179416. S2CID 7147886.
- Elliott J, Johnston A, Husereau D, Kelly SE, Eagles C, Charach A, et al. (21 October 2020). Gluud C (ed.). "Pharmacologic treatment of attention deficit hyperactivity disorder in adults: A systematic review and network meta-analysis". PLOS ONE. 15 (10). Public Library of Science (PLoS): e0240584. Bibcode:2020PLoSO..1540584E. doi:10.1371/journal.pone.0240584. PMC 7577505. PMID 33085721.
- Mohammadi MR, Kazemi MR, Zia E, Rezazadeh SA, Tabrizi M, Akhondzadeh S (November 2010). "Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double-blind trial". Human Psychopharmacology. 25 (7–8): 560–565. doi:10.1002/hup.1154. PMID 21312290. S2CID 30677758.
- Morrow K, Choi S, Young K, Haidar M, Boduch C, Bourgeois JA (September 2021). "Amantadine for the treatment of childhood and adolescent psychiatric symptoms". Proceedings. 34 (5): 566–570. doi:10.1080/08998280.2021.1925827. PMC 8366930. PMID 34456474.
- Gurnani T, Ivanov I, Newcorn JH (February 2016). "Pharmacotherapy of Aggression in Child and Adolescent Psychiatric Disorders". Journal of Child and Adolescent Psychopharmacology. 26 (1): 65–73. doi:10.1089/cap.2015.0167. PMID 26881859.
Several studies (e.g., Findling et al. 2000; Armenteros et al. 2007) have shown that antipsychotics, especially second generation agents, can be effective when used together with stimulants for aggression in ADHD
- Stevens JR, Wilens TE, Stern TA (2013). "Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges". The Primary Care Companion for CNS Disorders. 15 (2). doi:10.4088/PCC.12f01472. PMC 3733520. PMID 23930227.
- Young JL (20 December 2010). "Individualizing Treatment for Adult ADHD: An Evidence-Based Guideline". Medscape. Archived from the original on 8 May 2022. Retrieved 8 May 2022.
- Biederman J (21 November 2003). "New-Generation Long-Acting Stimulants for the Treatment of Attention-Deficit/Hyperactivity Disorder". Medscape. Archived from the original on 8 May 2022. Retrieved 8 May 2022.
As most treatment guidelines and prescribing information for stimulant medications relate to experience in school-aged children, prescribed doses for older patients are lacking. Emerging evidence for both methylphenidate and Adderall indicate that when weight-corrected daily doses, equipotent with those used in the treatment of younger patients, are used to treat adults with ADHD, these patients show a very robust clinical response consistent with that observed in pediatric studies. These data suggest that older patients may require a more aggressive approach in terms of dosing, based on the same target dosage ranges that have already been established – for methylphenidate, 1–1.5–2 mg/kg/day, and for D,L-amphetamine, 0.5–0.75–1 mg/kg/day....
In particular, adolescents and adults are vulnerable to underdosing, and are thus at potential risk of failing to receive adequate dosage levels. As with all therapeutic agents, the efficacy and safety of stimulant medications should always guide prescribing behavior: careful dosage titration of the selected stimulant product should help to ensure that each patient with ADHD receives an adequate dose, so that the clinical benefits of therapy can be fully attained. - Kessler S (January 1996). "Drug therapy in attention-deficit hyperactivity disorder". Southern Medical Journal. 89 (1): 33–38. doi:10.1097/00007611-199601000-00005. PMID 8545689. S2CID 12798818.
- ^ Den Heijer AE, Groen Y, Tucha L, Fuermaier AB, Koerts J, Lange KW, et al. (February 2017). "Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: a systematic literature review". Journal of Neural Transmission. 124 (Suppl 1): 3–26. doi:10.1007/s00702-016-1593-7. PMC 5281644. PMID 27400928.
Beneficial chronic effects of cardio exercise were found on various functions as well, including executive functions, attention and behavior.
- ^ Kamp CF, Sperlich B, Holmberg HC (July 2014). "Exercise reduces the symptoms of attention-deficit/hyperactivity disorder and improves social behaviour, motor skills, strength and neuropsychological parameters". Acta Paediatrica. 103 (7): 709–714. doi:10.1111/apa.12628. PMID 24612421. S2CID 45881887.
We may conclude that all different types of exercise ... attenuate the characteristic symptoms of ADHD and improve social behaviour, motor skills, strength and neuropsychological parameters without any undesirable side effects. Available reports do not reveal which type, intensity, duration and frequency of exercise is most effective
- ^ Rommel AS, Halperin JM, Mill J, Asherson P, Kuntsi J (September 2013). "Protection from genetic diathesis in attention-deficit/hyperactivity disorder: possible complementary roles of exercise". Journal of the American Academy of Child and Adolescent Psychiatry. 52 (9): 900–910. doi:10.1016/j.jaac.2013.05.018. PMC 4257065. PMID 23972692.
The findings from these studies provide some support for the notion that exercise has the potential to act as a protective factor for ADHD.
- Pelsser LM, Frankena K, Toorman J, Rodrigues Pereira R (January 2017). "Diet and ADHD, Reviewing the Evidence: A Systematic Review of Meta-Analyses of Double-Blind Placebo-Controlled Trials Evaluating the Efficacy of Diet Interventions on the Behavior of Children with ADHD". PLOS ONE (Systematic Review). 12 (1): e0169277. Bibcode:2017PLoSO..1269277P. doi:10.1371/journal.pone.0169277. PMC 5266211. PMID 28121994.
- Konikowska K, Regulska-Ilow B, Rózańska D (2012). "The influence of components of diet on the symptoms of ADHD in children". Roczniki Panstwowego Zakladu Higieny. 63 (2): 127–134. PMID 22928358.
- Arnold LE, DiSilvestro RA (August 2005). "Zinc in attention-deficit/hyperactivity disorder". Journal of Child and Adolescent Psychopharmacology. 15 (4): 619–627. doi:10.1089/cap.2005.15.619. hdl:1811/51593. PMID 16190793.
- Bloch MH, Mulqueen J (October 2014). "Nutritional supplements for the treatment of ADHD". Child and Adolescent Psychiatric Clinics of North America. 23 (4): 883–897. doi:10.1016/j.chc.2014.05.002. PMC 4170184. PMID 25220092.
- Krause J (April 2008). "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Review of Neurotherapeutics. 8 (4): 611–625. doi:10.1586/14737175.8.4.611. PMID 18416663. S2CID 24589993.
Zinc binds at ... extracellular sites of the DAT, serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc on symptoms of ADHD. It should be stated that at this time with zinc is not integrated in any ADHD treatment algorithm.
- Bálint S, Czobor P, Mészáros A, Simon V, Bitter I (2008). "A felnottkori figyelemhiányos/hiperaktivitás-zavarban tapasztalható neuropszichológiai deficit: irodalmi áttekintés" [Neuropsychological impairments in adult attention deficit hyperactivity disorder: A literature review]. Psychiatria Hungarica (in Hungarian). 23 (5). Magyar Pszichiátriai Társaság: 324–335. ISSN 0237-7896. PMID 19129549. PsycNET 2008-18348-001.
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. (August 2015). "Attention-deficit/hyperactivity disorder". Nature Reviews. Disease Primers (Review). 1: 15020. CiteSeerX 10.1.1.497.1346. doi:10.1038/nrdp.2015.20. PMID 27189265. S2CID 7171541.
- McClernon FJ, Kollins SH (October 2008). "ADHD and smoking: from genes to brain to behavior". Annals of the New York Academy of Sciences. 1141 (1): 131–147. Bibcode:2008NYASA1141..131M. doi:10.1196/annals.1441.016. PMC 2758663. PMID 18991955.
- ^ Ginsberg Y, Hirvikoski T, Lindefors N (December 2010). "Attention Deficit Hyperactivity Disorder (ADHD) among longer-term prison inmates is a prevalent, persistent and disabling disorder". BMC Psychiatry. 10 (1): 112. doi:10.1186/1471-244X-10-112. PMC 3016316. PMID 21176203.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Baggio S, Fructuoso A, Guimaraes M, Fois E, Golay D, Heller P, et al. (2 August 2018). "Prevalence of Attention Deficit Hyperactivity Disorder in Detention Settings: A Systematic Review and Meta-Analysis". Frontiers in Psychiatry. 9: 331. doi:10.3389/fpsyt.2018.00331. PMC 6084240. PMID 30116206.
- "State-based Prevalence Data of Parent Reported ADHD". Centers for Disease Control and Prevention. 13 February 2017. Archived from the original on 30 March 2019. Retrieved 31 March 2020.
- Willcutt EG (July 2012). "The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review". Neurotherapeutics. 9 (3): 490–499. doi:10.1007/s13311-012-0135-8. PMC 3441936. PMID 22976615.
- ^ Cowen P, Harrison P, Burns T (2012). "Drugs and other physical treatments". Shorter Oxford Textbook of Psychiatry (6th ed.). Oxford University Press. pp. 546. ISBN 978-0-19-960561-3 – via Google Books.
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (June 2007). "The worldwide prevalence of ADHD: a systematic review and metaregression analysis". The American Journal of Psychiatry. 164 (6): 942–948. doi:10.1176/appi.ajp.164.6.942. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183. PMID 17541055.
- Staller J, Faraone SV (2006). "Attention-deficit hyperactivity disorder in girls: epidemiology and management". CNS Drugs. 20 (2): 107–123. doi:10.2165/00023210-200620020-00003. PMID 16478287. S2CID 25835322.
- ^ "ADHD Throughout the Years" (PDF). Center For Disease Control and Prevention. Archived (PDF) from the original on 7 August 2013. Retrieved 2 August 2013.
- Connor DF (2011). "Problems of overdiagnosis and overprescribing in ADHD: are they legitimate?". Psychiatric Times. Vol. 28, no. 8. p. 14. Archived from the original on 12 August 2021.
- Dalsgaard S (February 2013). "Attention-deficit/hyperactivity disorder (ADHD)". European Child & Adolescent Psychiatry. 22 (Suppl 1): S43–S48. doi:10.1007/s00787-012-0360-z. PMID 23202886. S2CID 23349807.
- Coker TR, Elliott MN, Toomey SL, Schwebel DC, Cuccaro P, Tortolero Emery S, et al. (September 2016). "Racial and Ethnic Disparities in ADHD Diagnosis and Treatment". Pediatrics. 138 (3): e20160407. doi:10.1542/peds.2016-0407. PMC 5684883. PMID 27553219.
- ^ Slobodin O, Masalha R (June 2020). "Challenges in ADHD care for ethnic minority children: A review of the current literature". Transcultural Psychiatry. 57 (3): 468–483. doi:10.1177/1363461520902885. PMID 32233772. S2CID 214768588.
- Palmer ED, Finger S (May 2001). "An early description of ADHD (inattentive subtype): Dr Alexander Crichton and 'Mental restlessness' (1798)". Child and Adolescent Mental Health. 6 (2): 66–73. doi:10.1111/1475-3588.00324.
- Crichton A (1976) . An inquiry into the nature and origin of mental derangement: comprehending a concise system of the physiology and pathology of the human mind and a history of the passions and their effects. United Kingdom: AMS Press. p. 271. ISBN 978-0-404-08212-3. Archived from the original on 3 April 2019. Retrieved 17 January 2014 – via Google Books.
- Still G (1902). "Some Abnormal Psychical Conditions in Children: The Goulstonian Lectures". Lancet. 159: 1008–1012. doi:10.1016/s0140-6736(01)74984-7.
- ^ Rafalovich A (2001). "The Conceptual History of Attention Deficit Hyperactivity Disorder: Idiocy, Imbecility, Encephalitis and the Child Deviant". Deviant Behavior. 22: 93–115. doi:10.1080/016396201750065009. S2CID 43445475.
- Tredgold C (1908). Mental Deficiency: Amentia (1 ed.). New York: William Wood & Company. OCLC 990133. PsycNET 1908-10366-000. Archived from the original on 17 May 2022. Retrieved 17 May 2022.
- Connors C (2000). "Attention-Deficit/Hyperactivity Disorder: Historical Development and Overview". Journal of Attention Disorders: 173–191.
- Millichap JG (2010). "Definition and History of ADHD". Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). Springer Science. pp. 2–3. doi:10.1007/978-1-4419-1397-5_1. ISBN 978-1-4419-1396-8. LCCN 2009938108 – via Google Books.
- Weiss M, Hechtman LT, Weiss G (2001). "ADHD in Adulthood: An Introduction". ADHD in Adulthood: A Guide to Current Theory, Diagnosis, and Treatment. Taylor & Francis. pp. 34. ISBN 978-0-8018-6822-1 – via Google Books.
- Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929-1950". Journal of the History of Medicine and Allied Sciences. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800. S2CID 24974454.
- Patrick KS, Straughn AB, Perkins JS, González MA (January 2009). "Evolution of stimulants to treat ADHD: transdermal methylphenidate". Human Psychopharmacology. 24 (1): 1–17. doi:10.1002/hup.992. PMC 2629554. PMID 19051222.
- Gross MD (February 1995). "Origin of stimulant use for treatment of attention deficit disorder". The American Journal of Psychiatry. 152 (2): 298–299. doi:10.1176/ajp.152.2.298b. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183. PMID 7840374.
- Brown W (1998). "Charles Bradley, M.D.". American Journal of Psychiatry. 155 (7): 968. doi:10.1176/ajp.155.7.968. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183.
- ^ Barkley R (2006). Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: Guilford. pp. 42–5. ISBN 978-1-60623-750-2.
- Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT (July 1990). "Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder". Journal of the American Academy of Child and Adolescent Psychiatry. 29 (4): 526–533. doi:10.1097/00004583-199007000-00004. PMID 2387786.
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. (November 1994). "DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents". The American Journal of Psychiatry. 151 (11): 1673–1685. doi:10.1176/ajp.151.11.1673. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183. PMID 7943460.
- ^ Parrillo VN (2008). Encyclopedia of Social Problems. SAGE. p. 63. ISBN 9781412941655. Archived from the original on 4 January 2020. Retrieved 2 May 2009.
- Faraone SV (February 2005). "The scientific foundation for understanding attention-deficit/hyperactivity disorder as a valid psychiatric disorder". European Child & Adolescent Psychiatry. 14 (1): 1–10. doi:10.1007/s00787-005-0429-z. PMID 15756510. S2CID 143646869.
- Boseley S (30 September 2010). "Hyperactive children may have genetic disorder, says study". The Guardian. Archived from the original on 8 July 2017.
- ^ Cormier E (October 2008). "Attention deficit/hyperactivity disorder: a review and update". Journal of Pediatric Nursing. 23 (5): 345–357. doi:10.1016/j.pedn.2008.01.003. PMID 18804015.
- Schwarz A (14 December 2013). "The Selling of Attention Deficit Disorder". The New York Times. Archived from the original on 1 March 2015. Retrieved 26 February 2015.
- Elder TE (September 2010). "The importance of relative standards in ADHD diagnoses: evidence based on exact birth dates". Journal of Health Economics. 29 (5): 641–656. doi:10.1016/j.jhealeco.2010.06.003. PMC 2933294. PMID 20638739.
- ^ Ford-Jones PC (May 2015). "Misdiagnosis of attention deficit hyperactivity disorder: 'Normal behaviour' and relative maturity". Paediatrics & Child Health. 20 (4): 200–202. doi:10.1093/pch/20.4.200. PMC 4443828. PMID 26038639.
- Merten EC, Cwik JC, Margraf J, Schneider S (2017). "Overdiagnosis of mental disorders in children and adolescents (in developed countries)". Child and Adolescent Psychiatry and Mental Health. 11: 5. doi:10.1186/s13034-016-0140-5. PMC 5240230. PMID 28105068.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Taylor E (April 2017). "Attention deficit hyperactivity disorder: overdiagnosed or diagnoses missed?". Archives of Disease in Childhood. 102 (4): 376–379. doi:10.1136/archdischild-2016-310487. PMID 27821518. S2CID 19878394.
- "Are We Overdiagnosing and Overtreating ADHD?" Psychiatric Times, May 31, 2019. Rahil R. Jummani, MD. , Emily Hirsch, Glenn S. Hirsch, MD.
- Luan R, Mu Z, Yue F, He S (2017). "Efficacy and Tolerability of Different Interventions in Children and Adolescents with Attention Deficit Hyperactivity Disorder". Frontiers in Psychiatry. 8: 229. doi:10.3389/fpsyt.2017.00229. PMC 5694170. PMID 29180967.
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. (September 2018). "Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis". The Lancet. Psychiatry. 5 (9): 727–738. doi:10.1016/S2215-0366(18)30269-4. PMC 6109107. PMID 30097390.
- ^ Hoogman M, Stolte M, Baas M, Kroesbergen E (December 2020). "Creativity and ADHD: A review of behavioral studies, the effect of psychostimulants and neural underpinnings". Neuroscience and Biobehavioral Reviews. 119: 66–85. doi:10.1016/j.neubiorev.2020.09.029. PMID 33035524. S2CID 222142805.
- Catani M, Mazzarello P (June 2019). "Grey Matter Leonardo da Vinci: a genius driven to distraction". Brain. 142 (6): 1842–1846. doi:10.1093/brain/awz131. PMC 6536914. PMID 31121603.
- ^ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (October 2012). "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". Journal of the American Academy of Child and Adolescent Psychiatry. 51 (10): 1003–1019.e20. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
Further reading
- Hinshaw SP, Scheffler RM (2014). The ADHD Explosion: Myths, Medication, Money, and Today's Push for Performance. Oxford University Press. ISBN 978-0199790555.
- Reaser A, Prevatt F, Petscher Y, Proctor B (2007). "The learning and study strategies of college students with ADHD". Psychology in the Schools. 44 (6). Wiley-Blackwell: 627–638. doi:10.1002/pits.20252. eISSN 1520-6807. ISSN 0033-3085. LCCN 64009353. OCLC 1763062.
- Schwarz A (2016). ADHD Nation: Children, Doctors, Big Pharma, and the Making of an American Epidemic. Scribner. ISBN 978-1501105913. OCLC 951612166.
- Pliszka S (July 2007). "Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder". Journal of the American Academy of Child and Adolescent Psychiatry. 46 (7): 894–921. doi:10.1097/chi.0b013e318054e724. PMID 17581453. S2CID 602465.
External links
- National Institute of Mental Health. NIMH Pages About Attention-Deficit/Hyperactivity Disorder (ADHD). National Institutes of Health (NIH), U.S. Department of Health and Human Services. Archived 4 November 2021 at the Wayback Machine
- New Zealand Ministry of Health Guidelines for the Assessment and Treatment of Attention-Deficit/Hyperactivity Disorder. 2 July 2001. Archived 27 October 2014 at the Wayback Machine
- "Women and girls with ADHD" (video). (17 April 2020), with Stephen P. Hinshaw and others, Knowable Magazine Attention deficit hyperactivity disorder at the Internet Archive.
| Classification | D |
|---|---|
| External resources |
| Attention deficit hyperactivity disorder (ADHD) | |
|---|---|
| Main articles |
|
| Sub-types | |
| Medications |
|
| Related or outdated topics | |
| ADHD pharmacotherapies | |
|---|---|
| CNSTooltip central nervous system stimulants | |
| Non-classical CNS stimulants | |
| α2-adrenoceptor agonists | |
| Antidepressants | |
| Miscellaneous/others | |
| Related articles |
|
| Emotional and behavioral disorders | |
|---|---|
| Emotional/behavioral |
|
| Mental disorders (Classification) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| Digital media use and mental health | |
|---|---|
| Proposed or recognised diagnostic categories | |
| Disciplines involved | |
| Associated psychiatric conditions | |
| Related topics |
|