This is an old revision of this page, as edited by Chiswick Chap (talk | contribs) at 15:58, 6 January 2023 (→Water: trim). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:58, 6 January 2023 by Chiswick Chap (talk | contribs) (→Water: trim)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Science that studies life For other uses, see Biology (disambiguation). "Biological" redirects here. For other uses, see Biological (disambiguation).

Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary information encoded in genes, which can be transmitted to future generations. Another major theme is evolution, which explains the unity and diversity of life. Energy processing is also important to life as it allows organisms to move, grow, and reproduce. Finally, all organisms are able to regulate their own internal environments.
Biologists are able to study life at multiple levels of organization, from the molecular biology of a cell to the anatomy and physiology of plants and animals, and evolution of populations. Hence, there are multiple subdisciplines within biology, each defined by the nature of their research questions and the tools that they use. Like other scientists, biologists use the scientific method to make observations, pose questions, generate hypotheses, perform experiments, and form conclusions about the world around them.
Life on Earth, which emerged more than 3.7 billion years ago, is immensely diverse. Biologists have sought to study and classify the various forms of life, from prokaryotic organisms such as archaea and bacteria to eukaryotic organisms such as protists, fungi, plants, and animals. These various organisms contribute to the biodiversity of an ecosystem, where they play specialized roles in the cycling of nutrients and energy through their biophysical environment.
History
Further information: History of biology For a chronological guide, see Timeline of biology and organic chemistry.
The earliest of roots of science, which included medicine, can be traced to ancient Egypt and Mesopotamia in around 3000 to 1200 BCE. Their contributions later entered and shaped Greek natural philosophy of classical antiquity. Ancient Greek philosophers such as Aristotle (384–322 BCE) contributed extensively to the development of biological knowledge. His works such as History of Animals were especially important because they revealed his naturalist leanings, and later more empirical works that focused on biological causation and the diversity of life. Aristotle's successor at the Lyceum, Theophrastus, wrote a series of books on botany that survived as the most important contribution of antiquity to the plant sciences, even into the Middle Ages.
Scholars of the medieval Islamic world who wrote on biology included al-Jahiz (781–869), Al-Dīnawarī (828–896), who wrote on botany, and Rhazes (865–925) who wrote on anatomy and physiology. Medicine was especially well studied by Islamic scholars working in Greek philosopher traditions, while natural history drew heavily on Aristotelian thought, especially in upholding a fixed hierarchy of life.
Biology began to quickly develop and grow with Anton van Leeuwenhoek's dramatic improvement of the microscope. It was then that scholars discovered spermatozoa, bacteria, infusoria and the diversity of microscopic life. Investigations by Jan Swammerdam led to new interest in entomology and helped to develop the basic techniques of microscopic dissection and staining.
Advances in microscopy also had a profound impact on biological thinking. In the early 19th century, a number of biologists pointed to the central importance of the cell. Then, in 1838, Schleiden and Schwann began promoting the now universal ideas that (1) the basic unit of organisms is the cell and (2) that individual cells have all the characteristics of life, although they opposed the idea that (3) all cells come from the division of other cells. However, Robert Remak and Rudolf Virchow were able to reify the third tenet, and by the 1860s most biologists accepted all three tenets which consolidated into cell theory.
Meanwhile, taxonomy and classification became the focus of natural historians. Carl Linnaeus published a basic taxonomy for the natural world in 1735 (variations of which have been in use ever since), and in the 1750s introduced scientific names for all his species. Georges-Louis Leclerc, Comte de Buffon, treated species as artificial categories and living forms as malleable—even suggesting the possibility of common descent. Although he was opposed to evolution, Buffon is a key figure in the history of evolutionary thought; his work influenced the evolutionary theories of both Lamarck and Darwin.

Serious evolutionary thinking originated with the works of Jean-Baptiste Lamarck, who was the first to present a coherent theory of evolution. He posited that evolution was the result of environmental stress on properties of animals, meaning that the more frequently and rigorously an organ was used, the more complex and efficient it would become, thus adapting the animal to its environment. Lamarck believed that these acquired traits could then be passed on to the animal's offspring, who would further develop and perfect them. However, it was the British naturalist Charles Darwin, combining the biogeographical approach of Humboldt, the uniformitarian geology of Lyell, Malthus's writings on population growth, and his own morphological expertise and extensive natural observations, who forged a more successful evolutionary theory based on natural selection; similar reasoning and evidence led Alfred Russel Wallace to independently reach the same conclusions. Darwin's theory of evolution by natural selection quickly spread through the scientific community and soon became a central axiom of the rapidly developing science of biology.
The basis for modern genetics began with the work of Gregor Mendel, who presented his paper, "Versuche über Pflanzenhybriden" ("Experiments on Plant Hybridization"), in 1865, which outlined the principles of biological inheritance, serving as the basis for modern genetics. However, the significance of his work was not realized until the early 20th century when evolution became a unified theory as the modern synthesis reconciled Darwinian evolution with classical genetics. In the 1940s and early 1950s, a series of experiments by Alfred Hershey and Martha Chase pointed to DNA as the component of chromosomes that held the trait-carrying units that had become known as genes. A focus on new kinds of model organisms such as viruses and bacteria, along with the discovery of the double-helical structure of DNA by James Watson and Francis Crick in 1953, marked the transition to the era of molecular genetics. From the 1950s onwards, biology has been vastly extended in the molecular domain. The genetic code was cracked by Har Gobind Khorana, Robert W. Holley and Marshall Warren Nirenberg after DNA was understood to contain codons. Finally, the Human Genome Project was launched in 1990 with the goal of mapping the general human genome. This project was essentially completed in 2003, with further analysis still being published. The Human Genome Project was the first step in a globalized effort to incorporate accumulated knowledge of biology into a functional, molecular definition of the human body and the bodies of other organisms.
Chemical basis
Atoms and molecules
Further information: ChemistryAll organisms are made up of chemical elements; oxygen, carbon, hydrogen, and nitrogen account for 96% of all organisms, with calcium, phosphorus, sulfur, sodium, chlorine, and magnesium constituting essentially all the remainder. Different elements can combine to form compounds such as water, which is fundamental to life. Biochemistry is the study of chemical processes within and relating to living organisms. Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including molecular synthesis, modification, mechanisms, and interactions.
Water

Life arose from the Earth's first ocean, which formed some 3.8 billion years ago. Since then, water continues to be the most abundant molecule in every organism. Water is important to life because it is an effective solvent, capable of dissolving solutes such as sodium and chloride ions or other small molecules to form an aqueous solution. Once dissolved in water, these solutes are more likely to come in contact with one another and therefore take part in chemical reactions that sustain life. In terms of its molecular structure, water is a small polar molecule with a bent shape formed by the polar covalent bonds of two hydrogen (H) atoms to one oxygen (O) atom (H2O). Because the O–H bonds are polar, the oxygen atom has a slight negative charge and the two hydrogen atoms have a slight positive charge. This polar property of water allows it to attract other water molecules via hydrogen bonds, which makes water cohesive. Surface tension results from the cohesive force due to the attraction between molecules at the surface of the liquid. Water is also adhesive as it is able to adhere to the surface of any polar or charged non-water molecules. Water is denser as a liquid than it is as a solid (or ice). This unique property of water allows ice to float above liquid water such as ponds, lakes, and oceans, thereby insulating the liquid below from the cold air above. Water has the capacity to absorb energy, giving it a higher specific heat capacity than other solvents such as ethanol. Thus, a large amount of energy is needed to break the hydrogen bonds between water molecules to convert liquid water into water vapor. As a molecule, water is not completely stable as each water molecule continuously dissociates into hydrogen and hydroxyl ions before reforming into a water molecule again. In pure water, the number of hydrogen ions balances (or equals) the number of hydroxyl ions, resulting in a pH that is neutral.
Organic compounds
Further information: Organic chemistry
Organic compounds are molecules that contain carbon bonded to another element such as hydrogen. With the exception of water, nearly all the molecules that make up each organism contain carbon. Carbon can form covalent bonds with up to four other atoms, enabling it to form diverse, large, and complex molecules. For example, a single carbon atom can form four single covalent bonds such as in methane, two double covalent bonds such as in carbon dioxide (CO2), or a triple covalent bond such as in carbon monoxide (CO). Moreover, carbon can form very long chains of interconnecting carbon–carbon bonds such as octane or ring-like structures such as glucose.
The simplest form of an organic molecule is the hydrocarbon, which is a large family of organic compounds that are composed of hydrogen atoms bonded to a chain of carbon atoms. A hydrocarbon backbone can be substituted by other elements such as oxygen (O), hydrogen (H), phosphorus (P), and sulfur (S), which can change the chemical behavior of that compound. Groups of atoms that contain these elements (O-, H-, P-, and S-) and are bonded to a central carbon atom or skeleton are called functional groups. There are six prominent functional groups that can be found in organisms: amino group, carboxyl group, carbonyl group, hydroxyl group, phosphate group, and sulfhydryl group.
In 1953, the Miller-Urey experiment showed that organic compounds could be synthesized abiotically within a closed system mimicking the conditions of early Earth, thus suggesting that complex organic molecules could have arisen spontaneously in early Earth (see abiogenesis).
Macromolecules
Further information: Biochemistry, Macromolecule, and Molecular biology
Macromolecules are large molecules made up of smaller molecular subunits that are joined. Small molecules such as sugars, amino acids, and nucleotides can act as single repeating units called monomers to form chain-like molecules called polymers via a chemical process called condensation. For example, amino acids can form polypeptides whereas nucleotides can form strands of nucleic acid. Polymers make up three of the four macromolecules (polysaccharides, lipids, proteins, and nucleic acids) that are found in all organisms. Each of these macromolecules plays a specialized role within any given cell.
Carbohydrates (or sugar) are molecules with the molecular formula (CH2O)n, with n being the number of carbon-hydrate groups. They include monosaccharides (monomer), oligosaccharides (small polymers), and polysaccharides (large polymers). Monosaccharides can be linked together by glycosidic linkages, a type of covalent bond. When two monosaccharides such as glucose and fructose are linked together, they can form a disaccharide such as sucrose. When many monosaccharides are linked together, they can form an oligosaccharide or a polysaccharide, depending on the number of monosaccharides. Polysaccharides can vary in function. Monosaccharides such as glucose can be a source of energy and some polysaccharides can serve as storage material that can be hydrolyzed to provide cells with sugar.
Lipids are the only class of macromolecules that are not made up of polymers. The most biologically important lipids are steroids, phospholipids, and fats. These lipids are organic compounds that are largely nonpolar and hydrophobic. Steroids are organic compounds that consist of four fused rings. Phospholipids consist of glycerol that is linked to a phosphate group and two hydrocarbon chains (or fatty acids). The glycerol and phosphate group together constitute the polar and hydrophilic (or head) region of the molecule whereas the fatty acids make up the nonpolar and hydrophobic (or tail) region. Thus, when in water, phospholipids tend to form a phospholipid bilayer whereby the hydrophobic heads face outwards to interact with water molecules. Conversely, the hydrophobic tails face inwards towards other hydrophobic tails to avoid contact with water.
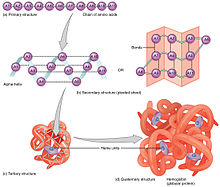
Proteins are the most diverse of the macromolecules, which include enzymes, transport proteins, large signaling molecules, antibodies, and structural proteins. The basic unit (or monomer) of a protein is an amino acid, which has a central carbon atom that is covalently bonded to a hydrogen atom, an amino group, a carboxyl group, and a side chain (or R-group, "R" for residue). There are twenty amino acids that make up the building blocks of proteins, with each amino acid having its own unique side chain. The polarity and charge of the side chains affect the solubility of amino acids. An amino acid with a side chain that is polar and electrically charged is soluble as it is hydrophilic whereas an amino acid with a side chain that lacks a charged or an electronegative atom is hydrophobic and therefore tends to coalesce rather than dissolve in water. Proteins have four distinct levels of organization (primary, secondary, tertiary, and quartenary). The primary structure consists of a unique sequence of amino acids that are covalently linked together by peptide bonds. The side chains of the individual amino acids can then interact with each other, giving rise to the secondary structure of a protein. The two common types of secondary structures are alpha helices and beta sheets. The folding of alpha helices and beta sheets gives a protein its three-dimensional or tertiary structure. Finally, multiple tertiary structures can combine to form the quaternary structure of a protein.
Nucleic acids are polymers made up of monomers called nucleotides. Their function is to store, transmit, and express hereditary information. Nucleotides consist of a phosphate group, a five-carbon sugar, and a nitrogenous base. Ribonucleotides, which contain ribose as the sugar, are the monomers of ribonucleic acid (RNA). In contrast, deoxyribonucleotides contain deoxyribose as the sugar and are constitute the monomers of deoxyribonucleic acid (DNA). RNA and DNA also differ with respect to one of their bases. There are two types of bases: purines and pyrimidines. The purines include guanine (G) and adenine (A) whereas the pyrimidines consist of cytosine (C), uracil (U), and thymine (T). Uracil is used in RNA whereas thymine is used in DNA. Taken together, when the different sugar and bases are take into consideration, there are eight distinct nucleotides that can form two types of nucleic acids: DNA (A, G, C, and T) and RNA (A, G, C, and U).
Cells
Further information: Cell biologyCell theory states that cells are the fundamental units of life, that all living things are composed of one or more cells, and that all cells arise from preexisting cells through cell division. Most cells are very small, with diameters ranging from 1 to 100 micrometers and are therefore only visible under a light or electron microscope. There are generally two types of cells: eukaryotic cells, which contain a nucleus, and prokaryotic cells, which do not. Prokaryotes are single-celled organisms such as bacteria, whereas eukaryotes can be single-celled or multicellular. In multicellular organisms, every cell in the organism's body is derived ultimately from a single cell in a fertilized egg.
Cell structure

Every cell is enclosed within a cell membrane that separates its cytoplasm from the extracellular space. A cell membrane consists of a lipid bilayer, including cholesterols that sit between phospholipids to maintain their fluidity at various temperatures. Cell membranes are semipermeable, allowing small molecules such as oxygen, carbon dioxide, and water to pass through while restricting the movement of larger molecules and charged particles such as ions. Cell membranes also contains membrane proteins, including integral membrane proteins that go across the membrane serving as membrane transporters, and peripheral proteins that loosely attach to the outer side of the cell membrane, acting as enzymes shaping the cell. Cell membranes are involved in various cellular processes such as cell adhesion, storing electrical energy, and cell signalling and serve as the attachment surface for several extracellular structures such as a cell wall, glycocalyx, and cytoskeleton.
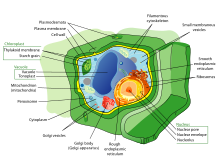
Within the cytoplasm of a cell, there are many biomolecules such as proteins and nucleic acids. In addition to biomolecules, eukaryotic cells have specialized structures called organelles that have their own lipid bilayers or are spatially units. These organelles include the cell nucleus, which contains most of the cell's DNA, or mitochondria, which generates adenosine triphosphate (ATP) to power cellular processes. Other organelles such as endoplasmic reticulum and Golgi apparatus play a role in the synthesis and packaging of proteins, respectively. Biomolecules such as proteins can be engulfed by lysosomes, another specialized organelle. Plant cells have additional organelles that distinguish them from animal cells such as a cell wall that provides support for the plant cell, chloroplasts that harvest sunlight energy to produce sugar, and vacuoles that provide storage and structural support as well as being involved in reproduction and breakdown of plant seeds. Eukaryotic cells also have cytoskeleton that is made up of microtubules, intermediate filaments, and microfilaments, all of which provide support for the cell and are involved in the movement of the cell and its organelles. In terms of their structural composition, the microtubules are made up of tubulin (e.g., α-tubulin and β-tubulin whereas intermediate filaments are made up of fibrous proteins. Microfilaments are made up of actin molecules that interact with other strands of proteins.
Metabolism
Further information: Bioenergetics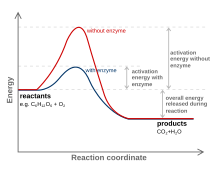
All cells require energy to sustain cellular processes. Energy is the capacity to do work, which, in thermodynamics, can be calculated using Gibbs free energy. According to the first law of thermodynamics, energy is conserved, i.e., cannot be created or destroyed. Hence, chemical reactions in a cell do not create new energy but are involved instead in the transformation and transfer of energy. Nevertheless, all energy transfers lead to some loss of usable energy, which increases entropy (or state of disorder) as stated by the second law of thermodynamics. As a result, an organism requires continuous input of energy to maintain a low state of entropy. In cells, energy can be transferred as electrons during redox (reduction–oxidation) reactions, stored in covalent bonds, and generated by the movement of ions (e.g., hydrogen, sodium, potassium) across a membrane.
Metabolism is the set of life-sustaining chemical reactions in organisms. The three main purposes of metabolism are: the conversion of food to energy to run cellular processes; the conversion of food/fuel to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolic reactions may be categorized as catabolic—the breaking down of compounds (for example, the breaking down of glucose to pyruvate by cellular respiration); or anabolic—the building up (synthesis) of compounds (such as proteins, carbohydrates, lipids, and nucleic acids). Usually, catabolism releases energy, and anabolism consumes energy.
The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, each step being facilitated by a specific enzyme. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts—they allow a reaction to proceed more rapidly without being consumed by it—by reducing the amount of activation energy needed to convert reactants into products. Enzymes also allow the regulation of the rate of a metabolic reaction, for example in response to changes in the cell's environment or to signals from other cells.
Cellular respiration
Further information: Cellular respiration
Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it clearly does not resemble one when it occurs in a cell because of the slow, controlled release of energy from the series of reactions.
Sugar in the form of glucose is the main nutrient used by animal and plant cells in respiration. Cellular respiration involving oxygen is called aerobic respiration, which has four stages: glycolysis, citric acid cycle (or Krebs cycle), electron transport chain, and oxidative phosphorylation. Glycolysis is a metabolic process that occurs in the cytoplasm whereby glucose is converted into two pyruvates, with two net molecules of ATP being produced at the same time. Each pyruvate is then oxidized into acetyl-CoA by the pyruvate dehydrogenase complex, which also generates NADH and carbon dioxide. Acetyl-Coa enters the citric acid cycle, which takes places inside the mitochondrial matrix. At the end of the cycle, the total yield from 1 glucose (or 2 pyruvates) is 6 NADH, 2 FADH2, and 2 ATP molecules. Finally, the next stage is oxidative phosphorylation, which in eukaryotes, occurs in the mitochondrial cristae. Oxidative phosphorylation comprises the electron transport chain, which is a series of four protein complexes that transfer electrons from one complex to another, thereby releasing energy from NADH and FADH2 that is coupled to the pumping of protons (hydrogen ions) across the inner mitochondrial membrane (chemiosmosis), which generates a proton motive force. Energy from the proton motive force drives the enzyme ATP synthase to synthesize more ATPs by phosphorylating ADPs. The transfer of electrons terminates with molecular oxygen being the final electron acceptor.
If oxygen were not present, pyruvate would not be metabolized by cellular respiration but undergoes a process of fermentation. The pyruvate is not transported into the mitochondrion but remains in the cytoplasm, where it is converted to waste products that may be removed from the cell. This serves the purpose of oxidizing the electron carriers so that they can perform glycolysis again and removing the excess pyruvate. Fermentation oxidizes NADH to NAD so it can be re-used in glycolysis. In the absence of oxygen, fermentation prevents the buildup of NADH in the cytoplasm and provides NAD for glycolysis. This waste product varies depending on the organism. In skeletal muscles, the waste product is lactic acid. This type of fermentation is called lactic acid fermentation. In strenuous exercise, when energy demands exceed energy supply, the respiratory chain cannot process all of the hydrogen atoms joined by NADH. During anaerobic glycolysis, NAD regenerates when pairs of hydrogen combine with pyruvate to form lactate. Lactate formation is catalyzed by lactate dehydrogenase in a reversible reaction. Lactate can also be used as an indirect precursor for liver glycogen. During recovery, when oxygen becomes available, NAD attaches to hydrogen from lactate to form ATP. In yeast, the waste products are ethanol and carbon dioxide. This type of fermentation is known as alcoholic or ethanol fermentation. The ATP generated in this process is made by substrate-level phosphorylation, which does not require oxygen.
Photosynthesis

Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that can later be released to fuel the organism's metabolic activities via cellular respiration. This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide and water. In most cases, oxygen is also released as a waste product. Most plants, algae, and cyanobacteria perform photosynthesis, which is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth.
Photosynthesis has four stages: Light absorption, electron transport, ATP synthesis, and carbon fixation. Light absorption is the initial step of photosynthesis whereby light energy is absorbed by chlorophyll pigments attached to proteins in the thylakoid membranes. The absorbed light energy is used to remove electrons from a donor (water) to a primary electron acceptor, a quinone designated as Q. In the second stage, electrons move from the quinone primary electron acceptor through a series of electron carriers until they reach a final electron acceptor, which is usually the oxidized form of NADP, which is reduced to NADPH, a process that takes place in a protein complex called photosystem I (PSI). The transport of electrons is coupled to the movement of protons (or hydrogen) from the stroma to the thylakoid membrane, which forms a pH gradient across the membrane as hydrogen becomes more concentrated in the lumen than in the stroma. This is analogous to the proton-motive force generated across the inner mitochondrial membrane in aerobic respiration.
During the third stage of photosynthesis, the movement of protons down their concentration gradients from the thylakoid lumen to the stroma through the ATP synthase is coupled to the synthesis of ATP by that same ATP synthase. The NADPH and ATPs generated by the light-dependent reactions in the second and third stages, respectively, provide the energy and electrons to drive the synthesis of glucose by fixing atmospheric carbon dioxide into existing organic carbon compounds, such as ribulose bisphosphate (RuBP) in a sequence of light-independent (or dark) reactions called the Calvin cycle.
Cell signaling
Further information: Cell signalingCell signaling (or communication) is the ability of cells to receive, process, and transmit signals with its environment and with itself. Signals can be non-chemical such as light, electrical impulses, and heat, or chemical signals (or ligands) that interact with receptors, which can be found embedded in the cell membrane of another cell or located deep inside a cell. There are generally four types of chemical signals: autocrine, paracrine, juxtacrine, and hormones. In autocrine signaling, the ligand affects the same cell that releases it. Tumor cells, for example, can reproduce uncontrollably because they release signals that initiate their own self-division. In paracrine signaling, the ligand diffuses to nearby cells and affects them. For example, brain cells called neurons release ligands called neurotransmitters that diffuse across a synaptic cleft to bind with a receptor on an adjacent cell such as another neuron or muscle cell. In juxtacrine signaling, there is direct contact between the signaling and responding cells. Finally, hormones are ligands that travel through the circulatory systems of animals or vascular systems of plants to reach their target cells. Once a ligand binds with a receptor, it can influence the behavior of another cell, depending on the type of receptor. For instance, neurotransmitters that bind with an inotropic receptor can alter the excitability of a target cell. Other types of receptors include protein kinase receptors (e.g., receptor for the hormone insulin) and G protein-coupled receptors. Activation of G protein-coupled receptors can initiate second messenger cascades. The process by which a chemical or physical signal is transmitted through a cell as a series of molecular events is called signal transduction
Cell cycle

The cell cycle is a series of events that take place in a cell that cause it to divide into two daughter cells. These events include the duplication of its DNA and some of its organelles, and the subsequent partitioning of its cytoplasm into two daughter cells in a process called cell division. In eukaryotes (i.e., animal, plant, fungal, and protist cells), there are two distinct types of cell division: mitosis and meiosis. Mitosis is part of the cell cycle, in which replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the total number of chromosomes is maintained. In general, mitosis (division of the nucleus) is preceded by the S stage of interphase (during which the DNA is replicated) and is often followed by telophase and cytokinesis; which divides the cytoplasm, organelles and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis all together define the mitotic phase of an animal cell cycle—the division of the mother cell into two genetically identical daughter cells. The cell cycle is a vital process by which a single-celled fertilized egg develops into a mature organism, as well as the process by which hair, skin, blood cells, and some internal organs are renewed. After cell division, each of the daughter cells begin the interphase of a new cycle. In contrast to mitosis, meiosis results in four haploid daughter cells by undergoing one round of DNA replication followed by two divisions. Homologous chromosomes are separated in the first division (meiosis I), and sister chromatids are separated in the second division (meiosis II). Both of these cell division cycles are used in the process of sexual reproduction at some point in their life cycle. Both are believed to be present in the last eukaryotic common ancestor.
Prokaryotes (i.e., archaea and bacteria) can also undergo cell division (or binary fission). Unlike the processes of mitosis and meiosis in eukaryotes, binary fission takes in prokaryotes takes place without the formation of a spindle apparatus on the cell. Before binary fission, DNA in the bacterium is tightly coiled. After it has uncoiled and duplicated, it is pulled to the separate poles of the bacterium as it increases the size to prepare for splitting. Growth of a new cell wall begins to separate the bacterium (triggered by FtsZ polymerization and "Z-ring" formation) The new cell wall (septum) fully develops, resulting in the complete split of the bacterium. The new daughter cells have tightly coiled DNA rods, ribosomes, and plasmids.
Genetics
Inheritance
Further information: Classical genetics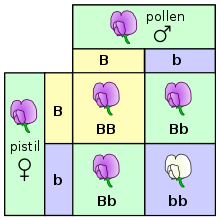
Genetics is the scientific study of inheritance. Mendelian inheritance, specifically, is the process by which genes and traits are passed on from parents to offspring. It was formulated by Gregor Mendel, based on his work with pea plants in the mid-nineteenth century. Mendel established several principles of inheritance. The first is that genetic characteristics, which are now called alleles, are discrete and have alternate forms (e.g., purple vs. white or tall vs. dwarf), each inherited from one of two parents. Based on his law of dominance and uniformity, which states that some alleles are dominant while others are recessive; an organism with at least one dominant allele will display the phenotype of that dominant allele. Exceptions to this rule include penetrance and expressivity. Mendel noted that during gamete formation, the alleles for each gene segregate from each other so that each gamete carries only one allele for each gene, which is stated by his law of segregation. Heterozygotic individuals produce gametes with an equal frequency of two alleles. Finally, Mendel formulated the law of independent assortment, which states that genes of different traits can segregate independently during the formation of gametes, i.e., genes are unlinked. An exception to this rule would include traits that are sex-linked. Test crosses can be performed to experimentally determine the underlying genotype of an organism with a dominant phenotype. A Punnett square can be used to predict the results of a test cross. The chromosome theory of inheritance, which states that genes are found on chromosomes, was supported by Thomas Morgans's experiments with fruit flies, which established the sex linkage between eye color and sex in these insects. In humans and other mammals (e.g., dogs), it is not feasible or practical to conduct test cross experiments. Instead, pedigrees, which are genetic representations of family trees, are used instead to trace the inheritance of a specific trait or disease through multiple generations.
DNA

A gene is a unit of heredity that corresponds to a region of deoxyribonucleic acid (DNA) that carries genetic information that controls form or function of an organism. DNA is composed of two polynucleotide chains that coil around each other to form a double helix. It is found as linear chromosomes in eukaryotes, and circular chromosomes in prokaryotes. The set of chromosomes in a cell is collectively known as its genome. In eukaryotes, DNA is mainly in the cell nucleus. In prokaryotes, the DNA is held within the nucleoid. The genetic information is held within genes, and the complete assemblage in an organism is called its genotype. DNA replication is a semiconservative process whereby each strand serves as a template for a new strand of DNA. Mutations are heritable changes in DNA. They can arise spontaneously as a result of replication errors that were not corrected by proofreading or can be induced by an environmental mutagen such as a chemical (e.g., nitrous acid, benzopyrene) or radiation (e.g., x-ray, gamma ray, ultraviolet radiation, particles emitted by unstable isotopes). Mutations can lead to phenotypic effects such as loss-of-function, gain-of-function, and conditional mutations. Some mutations are beneficial, as they are a source of genetic variation for evolution. Others are harmful if they were to result in a loss of function of genes needed for survival. Mutagens such as carcinogens are typically avoided as a matter of public health policy goals.
Gene expression

Gene expression is the molecular process by which a genotype encoded in DNA gives rise to an observable phenotype in the proteins of an organism's body. This process is summarized by the central dogma of molecular biology, which was formulated by Francis Crick in 1958. According to the Central Dogma, genetic information flows from DNA to RNA to protein. There are two gene expression processes: transcription (DNA to RNA) and translation (RNA to protein).
Gene regulation
Main article: Regulation of gene expressionThe regulation of gene expression by environmental factors and during different stages of development can occur at each step of the process such as transcription, RNA splicing, translation, and post-translational modification of a protein. Gene expression can be influenced by positive or negative regulation, depending on which of the two types of regulatory proteins called transcription factors bind to the DNA sequence close to or at a promoter. A cluster of genes that share the same promoter is called an operon, found mainly in prokaryotes and some lower eukaryotes (e.g., Caenorhabditis elegans). In positive regulation of gene expression, the activator is the transcription factor that stimulates transcription when it binds to the sequence near or at the promoter. Negative regulation occurs when another transcription factor called a repressor binds to a DNA sequence called an operator, which is part of an operon, to prevent transcription. Repressors can be inhibited by compounds called inducers (e.g., allolactose), thereby allowing transcription to occur. Specific genes that can be activated by inducers are called inducible genes, in contrast to constitutive genes that are almost constantly active. In contrast to both, structural genes encode proteins that are not involved in gene regulation. In addition to regulatory events involving the promoter, gene expression can also be regulated by epigenetic changes to chromatin, which is a complex of DNA and protein found in eukaryotic cells.
Genes, development, and evolution
Further information: Evolutionary developmental biologyDevelopment is the process by which a multicellular organism (plant or animal) goes through a series of changes, starting from a single cell, and taking on various forms that are characteristic of its life cycle. There are four key processes that underlie development: Determination, differentiation, morphogenesis, and growth. Determination sets the developmental fate of a cell, which becomes more restrictive during development. Differentiation is the process by which specialized cells from less specialized cells such as stem cells. Stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. Cellular differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals, which are largely due to highly controlled modifications in gene expression and epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Thus, different cells can have very different physical characteristics despite having the same genome. Morphogenesis, or the development of body form, is the result of spatial differences in gene expression. A small fraction of the genes in an organism's genome called the developmental-genetic toolkit control the development of that organism. These toolkit genes are highly conserved among phyla, meaning that they are ancient and very similar in widely separated groups of animals. Differences in deployment of toolkit genes affect the body plan and the number, identity, and pattern of body parts. Among the most important toolkit genes are the Hox genes. Hox genes determine where repeating parts, such as the many vertebrae of snakes, will grow in a developing embryo or larva.
Evolution
Evolutionary processes
Further information: Evolutionary biology
A central organizing concept in biology is that life changes and develops through evolution, which is the change in heritable characteristics of populations over successive generations. Evolution is now used to explain the great variations of life on Earth. The term evolution was introduced into the scientific lexicon by Jean-Baptiste de Lamarck in 1809. He proposed that evolution occurred as a result of inheritance of acquired characteristics, which was unconvincing but there were no alternative explanations at the time. Charles Darwin, an English naturalist, had returned to England in 1836 from his five-year travels on the HMS Beagle where he studied rocks and collected plants and animals from various parts of the world such as the Galápagos Islands. He had also read Principles of Geology by Charles Lyell and An Essay on the Principle of Population by Thomas Malthus and was influenced by them. Based on his observations and readings, Darwin began to formulate his theory of evolution by natural selection to explain the diversity of plants and animals in different parts of the world. Alfred Russel Wallace, another English naturalist who had studied plants and animals in the Malay Archipelago, also came to the same idea, but later and independently of Darwin. Both Darwin and Wallace jointly presented their essay and manuscript, respectively, at the Linnaean Society of London in 1858, giving them both credit for their discovery of evolution by natural selection. Darwin would later publish his book On the Origin of Species in 1859, which explained in detail how the process of evolution by natural selection works.
To explain natural selection, Darwin drew an analogy with humans modifying animals through artificial selection, whereby animals were selectively bred for specific traits, which has given rise to individuals that no longer resemble their wild ancestors. Darwin argued that in the natural world, it was nature that played the role of humans in selecting for specific traits. He came to this conclusion based on two observations and two inferences. First, members of any population tend to vary with respect to their heritable traits. Second, all species tend to produce more offspring than can be supported by their respective environments, resulting in many individuals not surviving and reproducing. Based on these observations, Darwin inferred that those individuals who possessed heritable traits that are better adapted to their environments are more likely to survive and produce more offspring than other individuals. He further inferred that the unequal or differential survival and reproduction of certain individuals over others will lead to the accumulation of favorable traits over successive generations, thereby increasing the match between the organisms and their environment. Thus, taken together, natural selection is the differential survival and reproduction of individuals in subsequent generations due to differences in or more heritable traits.
Darwin was not aware of Mendel's work of inheritance and so the exact mechanism of inheritance that underlie natural selection was not well-understood until the early 20th century when the modern synthesis reconciled Darwinian evolution with classical genetics, which established a neo-Darwinian perspective of evolution by natural selection. This perspective holds that evolution occurs when there are changes in the allele frequencies within a population of interbreeding organisms. In the absence of any evolutionary process acting on a large random mating population, the allele frequencies will remain constant across generations as described by the Hardy–Weinberg principle.
Another process that drives evolution is genetic drift, which is the random fluctuations of allele frequencies within a population from one generation to the next. When selective forces are absent or relatively weak, allele frequencies are equally likely to drift upward or downward at each successive generation because the alleles are subject to sampling error. This drift halts when an allele eventually becomes fixed, either by disappearing from the population or replacing the other alleles entirely. Genetic drift may therefore eliminate some alleles from a population due to chance alone.
Speciation

A species is a group of organisms that mate with one another and speciation is the process by which one lineage splits into two lineages as a result of having evolved independently from each other. For speciation to occur, there has to be reproductive isolation. Reproductive isolation can result from incompatibilities between genes as described by Bateson–Dobzhansky–Muller model. Reproductive isolation also tends to increase with genetic divergence. Speciation can occur when there are physical barriers that divide an ancestral species, a process known as allopatric speciation. In contrast, sympatric speciation occurs in the absence of physical barriers.
Pre-zygotic isolation such as mechanical, temporal, behavioral, habitat, and gametic isolations can prevent different species from hybridizing. Similarly, post-zygotic isolations can result in hybridization being selected against due to the lower viability of hybrids or hybrid infertility (e.g., mule). Hybrid zones can emerge if there were to be incomplete reproductive isolation between two closely related species.
Phylogeny
Further information: Phylogenetics and Biodiversity
A phylogeny is an evolutionary history of a specific group of organisms or their genes. It can be represented using a phylogenetic tree, which is a diagram showing lines of descent among organisms or their genes. Each line drawn on the time axis of a tree represents a lineage of descendants of a particular species or population. When a lineage divides into two, it is represented as a node (or split) on the phylogenetic tree. The more splits there are over time, the more branches there will be on the tree, with the common ancestor of all the organisms in that tree being represented by the root of that tree. Phylogenetic trees may portray the evolutionary history of all life forms, a major evolutionary group (e.g., insects), or an even smaller group of closely related species. Within a tree, any group of species designated by a name is a taxon (e.g., humans, primates, mammals, or vertebrates) and a taxon that consists of all its evolutionary descendants is a clade, otherwise known as a monophyletic taxon. Closely related species are referred to as sister species and closely related clades are sister clades. In contrast to a monophyletic group, a polyphyletic group does not include its common ancestor whereas a paraphyletic group does not include all the descendants of a common ancestor.
Phylogenetic trees are the basis for comparing and grouping different species. Different species that share a feature inherited from a common ancestor are described as having homologous features (or synapomorphy). Homologous features may be any heritable traits such as DNA sequence, protein structures, anatomical features, and behavior patterns. A vertebral column is an example of a homologous feature shared by all vertebrate animals. Traits that have a similar form or function but were not derived from a common ancestor are described as analogous features. Phylogenies can be reconstructed for a group of organisms of primary interests, which are called the ingroup. A species or group that is closely related to the ingroup but is phylogenetically outside of it is called the outgroup, which serves a reference point in the tree. The root of the tree is located between the ingroup and the outgroup. When phylogenetic trees are reconstructed, multiple trees with different evolutionary histories can be generated. Based on the principle of Parsimony (or Occam's razor), the tree that is favored is the one with the fewest evolutionary changes needed to be assumed over all traits in all groups. Computational algorithms can be used to determine how a tree might have evolved given the evidence.
Phylogeny provides the basis of biological classification, which is based on Linnaean taxonomy that was developed by Carl Linnaeus in the 18th century. This classification system is rank-based, with the highest rank being the domain followed by kingdom, phylum, class, order, family, genus, and species. All organisms can be classified as belonging to one of three domains: Archaea (originally Archaebacteria); bacteria (originally eubacteria), or eukarya (includes the protist, fungi, plant, and animal kingdoms). A binomial nomenclature is used to classify different species. Based on this system, each species is given two names, one for its genus and another for its species. For example, humans are Homo sapiens, with Homo being the genus and sapiens being the species. By convention, the scientific names of organisms are italicized, with only the first letter of the genus capitalized.
History of life
| Life timeline | ||||||||||||||||||||||||||||||||||||||||||||||
| This box: | ||||||||||||||||||||||||||||||||||||||||||||||
| −4500 —–—–−4000 —–—–−3500 —–—–−3000 —–—–−2500 —–—–−2000 —–—–−1500 —–—–−1000 —–—–−500 —–—–0 — | Water Single-celled life Photosynthesis Eukaryotes Multicellular life P l a n t s Arthropods MolluscsFlowersDinosaurs MammalsBirdsPrimatesH a d e a n A r c h e a n P r o t e r o z o i cP h a n e r o z o i c |
| ||||||||||||||||||||||||||||||||||||||||||||
| (million years ago)*Ice Ages | ||||||||||||||||||||||||||||||||||||||||||||||
The history of life on Earth traces the processes by which organisms have evolved from the earliest emergence of life to present day. Earth formed about 4.5 billion years ago and all life on Earth, both living and extinct, descended from a last universal common ancestor that lived about 3.5 billion years ago. The dating of the Earth's history can be done using several geological methods such as stratigraphy, radiometric dating, and paleomagnetic dating. Based on these methods, geologists have developed a geologic time scale that divides the history of the Earth into major divisions, starting with four eons (Hadean, Archean, Proterozoic, and Phanerozoic), the first three of which are collectively known as the Precambrian, which lasted approximately 4 billion years. Each eon can be divided into eras, with the Phanerozoic eon that began 539 million years ago being subdivided into Paleozoic, Mesozoic, and Cenozoic eras. These three eras together comprise eleven periods (Cambrian, Ordovician, Silurian, Devonian, Carboniferous, Permian, Triassic, Jurassic, Cretaceous, Tertiary, and Quaternary) and each period into epochs.
The similarities among all known present-day species indicate that they have diverged through the process of evolution from their common ancestor. Biologists regard the ubiquity of the genetic code as evidence of universal common descent for all bacteria, archaea, and eukaryotes. Microbal mats of coexisting bacteria and archaea were the dominant form of life in the early Archean epoch and many of the major steps in early evolution are thought to have taken place in this environment. The earliest evidence of eukaryotes dates from 1.85 billion years ago, and while they may have been present earlier, their diversification accelerated when they started using oxygen in their metabolism. Later, around 1.7 billion years ago, multicellular organisms began to appear, with differentiated cells performing specialised functions.
Algae-like multicellular land plants are dated back even to about 1 billion years ago, although evidence suggests that microorganisms formed the earliest terrestrial ecosystems, at least 2.7 billion years ago. Microorganisms are thought to have paved the way for the inception of land plants in the Ordovician period. Land plants were so successful that they are thought to have contributed to the Late Devonian extinction event.
Ediacara biota appear during the Ediacaran period, while vertebrates, along with most other modern phyla originated about 525 million years ago during the Cambrian explosion. During the Permian period, synapsids, including the ancestors of mammals, dominated the land, but most of this group became extinct in the Permian–Triassic extinction event 252 million years ago. During the recovery from this catastrophe, archosaurs became the most abundant land vertebrates; one archosaur group, the dinosaurs, dominated the Jurassic and Cretaceous periods. After the Cretaceous–Paleogene extinction event 66 million years ago killed off the non-avian dinosaurs, mammals increased rapidly in size and diversity. Such mass extinctions may have accelerated evolution by providing opportunities for new groups of organisms to diversify.
Diversity
Bacteria and Archaea
Further information: Microbiology
Bacteria are a type of cell that constitute a large domain of prokaryotic microorganisms. Typically a few micrometers in length, bacteria have a number of shapes, ranging from spheres to rods and spirals. Bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of the earth's crust. Bacteria also live in symbiotic and parasitic relationships with plants and animals. Most bacteria have not been characterised, and only about 27 percent of the bacterial phyla have species that can be grown in the laboratory.
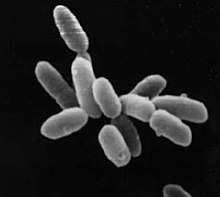
Archaea constitute the other domain of prokaryotic cells and were initially classified as bacteria, receiving the name archaebacteria (in the Archaebacteria kingdom), a term that has fallen out of use. Archaeal cells have unique properties separating them from the other two domains, Bacteria and Eukaryota. Archaea are further divided into multiple recognized phyla. Archaea and bacteria are generally similar in size and shape, although a few archaea have very different shapes, such as the flat and square cells of Haloquadratum walsbyi. Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably for the enzymes involved in transcription and translation. Other aspects of archaeal biochemistry are unique, such as their reliance on ether lipids in their cell membranes, including archaeols. Archaea use more energy sources than eukaryotes: these range from organic compounds, such as sugars, to ammonia, metal ions or even hydrogen gas. Salt-tolerant archaea (the Haloarchaea) use sunlight as an energy source, and other species of archaea fix carbon, but unlike plants and cyanobacteria, no known species of archaea does both. Archaea reproduce asexually by binary fission, fragmentation, or budding; unlike bacteria, no known species of Archaea form endospores.
The first observed archaea were extremophiles, living in extreme environments, such as hot springs and salt lakes with no other organisms. Improved molecular detection tools led to the discovery of archaea in almost every habitat, including soil, oceans, and marshlands. Archaea are particularly numerous in the oceans, and the archaea in plankton may be one of the most abundant groups of organisms on the planet.
Archaea are a major part of Earth's life. They are part of the microbiota of all organisms. In the human microbiome, they are important in the gut, mouth, and on the skin. Their morphological, metabolic, and geographical diversity permits them to play multiple ecological roles: carbon fixation; nitrogen cycling; organic compound turnover; and maintaining microbial symbiotic and syntrophic communities, for example.
Eukaryotes
Main article: Eukaryote
Eukaryotes are hypothesized to have split from archaea, which was followed by their endosymbioses with bacteria (or symbiogenesis) that gave rise to mitochondria and chloroplasts, both of which are now part of modern-day eukaryotic cells. The major lineages of eukaryotes diversified in the Precambrian about 1.5 billion years ago and can be classified into eight major clades: alveolates, excavates, stramenopiles, plants, rhizarians, amoebozoans, fungi, and animals. Five of these clades are collectively known as protists, which are mostly microscopic eukaryotic organisms that are not plants, fungi, or animals. While it is likely that protists share a common ancestor (the last eukaryotic common ancestor), protists by themselves do not constitute a separate clade as some protists may be more closely related to plants, fungi, or animals than they are to other protists. Like groupings such as algae, invertebrates, or protozoans, the protist grouping is not a formal taxonomic group but is used for convenience. Most protists are unicellular; these are called microbial eukaryotes.
Plants are mainly multicellular organisms, predominantly photosynthetic eukaryotes of the kingdom Plantae, which would exclude fungi and some algae. Plant cells were derived by endosymbiosis of a cyanobacterium into an early eukaryote about one billion years ago, which gave rise to chloroplasts. The first several clades that emerged following primary endosymbiosis were aquatic and most of the aquatic photosynthetic eukaryotic organisms are collectively described as algae, which is a term of convenience as not all algae are closely related. Algae comprise several distinct clades such as glaucophytes, which are microscopic freshwater algae that may have resembled in form to the early unicellular ancestor of Plantae. Unlike glaucophytes, the other algal clades such as red and green algae are multicellular. Green algae comprise three major clades: chlorophytes, coleochaetophytes, and stoneworts.
Fungi are eukaryotes that digest foods outside their bodies, secreting digestive enzymes that break down large food molecules before absorbing them through their cell membranes. Many fungi are also saprobes, feeding on dead organic matter, making them important decomposers in ecological systems.
Animals are multicellular eukaryotes. With few exceptions, animals consume organic material, breathe oxygen, are able to move, can reproduce sexually, and grow from a hollow sphere of cells, the blastula, during embryonic development. Over 1.5 million living animal species have been described—of which around 1 million are insects—but it has been estimated there are over 7 million animal species in total. They have complex interactions with each other and their environments, forming intricate food webs.
Viruses
Further information: Virology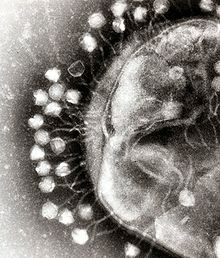
Viruses are submicroscopic infectious agents that replicate inside the cells of organisms. Viruses infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea. More than 6,000 virus species have been described in detail. Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity.
The origins of viruses in the evolutionary history of life are unclear: some may have evolved from plasmids—pieces of DNA that can move between cells—while others may have evolved from bacteria. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity in a way analogous to sexual reproduction. Because viruses possess some but not all characteristics of life, they have been described as "organisms at the edge of life", and as self-replicators.
Ecology
Main article: EcologyEcology is the study of the distribution and abundance of life, the interaction between organisms and their environment.
Ecosystems
Main article: Ecosystem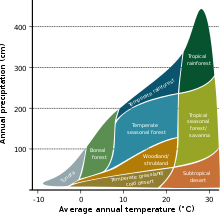
The community of living (biotic) organisms in conjunction with the nonliving (abiotic) components (e.g., water, light, radiation, temperature, humidity, atmosphere, acidity, and soil) of their environment is called an ecosystem. These biotic and abiotic components are linked together through nutrient cycles and energy flows. Energy from the sun enters the system through photosynthesis and is incorporated into plant tissue. By feeding on plants and on one another, animals play an important role in the movement of matter and energy through the system. They also influence the quantity of plant and microbial biomass present. By breaking down dead organic matter, decomposers release carbon back to the atmosphere and facilitate nutrient cycling by converting nutrients stored in dead biomass back to a form that can be readily used by plants and other microbes.
The Earth's physical environment is shaped by solar energy and topography. The amount of solar energy input varies in space and time due to the spherical shape of the Earth and its axial tilt. Variation in solar energy input drives weather and climate patterns. Weather is the day-to-day temperature and precipitation activity, whereas climate is the long-term average of weather, typically averaged over a period of 30 years. Variation in topography also produces environmental heterogeneity. On the windward side of a mountain, for example, air rises and cools, with water changing from gaseous to liquid or solid form, resulting in precipitation such as rain or snow. As a result, wet environments allow for lush vegetation to grow. In contrast, conditions tend to be dry on the leeward side of a mountain due to the lack of precipitation as air descends and warms, and moisture remains as water vapor in the atmosphere. Temperature and precipitation are the main factors that shape terrestrial biomes.
Populations
Further information: Population ecology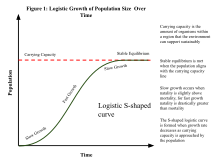
A population is the number of organisms of the same species that occupy an area and reproduce from generation to generation. Its abundance can be measured using population density, which is the number of individuals per unit area (e.g., land or tree) or volume (e.g., sea or air). Given that it is usually impractical to count every individual within a large population to determine its size, population size can be estimated by multiplying population density by the area or volume. Population growth during short-term intervals can be determined using the population growth rate equation, which takes into consideration birth, death, and immigration rates. In the longer term, the exponential growth of a population tends to slow down as it reaches its carrying capacity, which can be modeled using the logistic equation. The carrying capacity of an environment is the maximum population size of a species that can be sustained by that specific environment, given the food, habitat, water, and other resources that are available. The carrying capacity of a population can be affected by changing environmental conditions such as changes in the availability resources and the cost of maintaining them. In human populations, new technologies such as the Green revolution have helped increase the Earth's carrying capacity for humans over time, which has stymied the attempted predictions of impending population decline, the famous of which was by Thomas Malthus in the 18th century.
Communities
Main article: Community (ecology)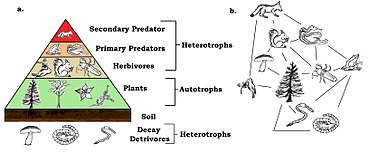
A community is a group of populations of two or more different species occupying the same geographical area at the same time. A biological interaction is the effect that a pair of organisms living together in a community have on each other. They can be either of the same species (intraspecific interactions), or of different species (interspecific interactions). These effects may be short-term, like pollination and predation, or long-term; both often strongly influence the evolution of the species involved. A long-term interaction is called a symbiosis. Symbioses range from mutualism, beneficial to both partners, to competition, harmful to both partners.
Every species participates as a consumer, resource, or both in consumer–resource interactions, which form the core of food chains or food webs. There are different trophic levels within any food web, with the lowest level being the primary producers (or autotrophs) such as plants and algae that convert energy and inorganic material into organic compounds, which can then be used by the rest of the community. At the next level are the heterotrophs, which are the species that obtain energy by breaking apart organic compounds from other organisms. Heterotrophs that consume plants are primary consumers (or herbivores) whereas heterotrophs that consume herbivores are secondary consumers (or carnivores). And those that eat secondary consumers are tertiary consumers and so on. Omnivorous heterotrophs are able to consume at multiple levels. Finally, there are decomposers that feed on the waste products or dead bodies of organisms.
On average, the total amount of energy incorporated into the biomass of a trophic level per unit of time is about one-tenth of the energy of the trophic level that it consumes. Waste and dead material used by decomposers as well as heat lost from metabolism make up the other ninety percent of energy that is not consumed by the next trophic level.
Biosphere
Main article: Biosphere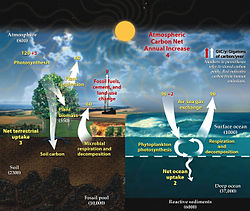
In the global ecosystem (or biosphere), matter exist as different interacting compartments, which can be biotic or abiotic as well as accessible or inaccessible, depending on their forms and locations. For example, matter from terrestrial autotrophs are both biotic and accessible to other organisms whereas the matter in rocks and minerals are abiotic and inaccessible. A biogeochemical cycle is a pathway by which specific elements of matter are turned over or moved through the biotic (biosphere) and the abiotic (lithosphere, atmosphere, and hydrosphere) compartments of Earth. There are biogeochemical cycles for nitrogen, carbon, and water. In some cycles there are reservoirs where a substance remains or is sequestered for a long period of time.
Climate change includes both global warming driven by human-induced emissions of greenhouse gases and the resulting large-scale shifts in weather patterns. Though there have been previous periods of climatic change, since the mid-20th century humans have had an unprecedented impact on Earth's climate system and caused change on a global scale. The largest driver of warming is the emission of greenhouse gases, of which more than 90% are carbon dioxide and methane. Fossil fuel burning (coal, oil, and natural gas) for energy consumption is the main source of these emissions, with additional contributions from agriculture, deforestation, and manufacturing. Temperature rise is accelerated or tempered by climate feedbacks, such as loss of sunlight-reflecting snow and ice cover, increased water vapor (a greenhouse gas itself), and changes to land and ocean carbon sinks.
Conservation
Main article: Conservation biologyConservation biology is the study of the conservation of Earth's biodiversity with the aim of protecting species, their habitats, and ecosystems from excessive rates of extinction and the erosion of biotic interactions. It is concerned with factors that influence the maintenance, loss, and restoration of biodiversity and the science of sustaining evolutionary processes that engender genetic, population, species, and ecosystem diversity. The concern stems from estimates suggesting that up to 50% of all species on the planet will disappear within the next 50 years, which has contributed to poverty, starvation, and will reset the course of evolution on this planet. Biodiversity affects the functioning of ecosystems, which provide a variety of services upon which people depend.
Conservation biologists research and educate on the trends of biodiversity loss, species extinctions, and the negative effect these are having on our capabilities to sustain the well-being of human society. Organizations and citizens are responding to the current biodiversity crisis through conservation action plans that direct research, monitoring, and education programs that engage concerns at local through global scales.
See also
- Biology in fiction
- Glossary of biology
- List of biological websites
- List of biologists
- List of biology journals
- List of biology topics
- List of life sciences
- List of omics topics in biology
- National Association of Biology Teachers
- Outline of biology
- Periodic table of life sciences in Tinbergen's four questions
- Reproduction
- Science tourism
- Terminology of biology
References
- ^ Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "Evolution, the themes of biology, and scientific inquiry". Campbell Biology (11th ed.). New York: Pearson. pp. 2–26. ISBN 978-0134093413.
- ^ Hillis, David M.; Heller, H. Craig; Hacker, Sally D.; Laskowski, Marta J.; Sadava, David E. (2020). "Studying life". Life: The Science of Biology (12th ed.). W. H. Freeman. ISBN 978-1319017644.
- ^ Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Biology and the three of life". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 1–18. ISBN 978-0321976499.
- Modell, Harold; Cliff, William; Michael, Joel; McFarland, Jenny; Wenderoth, Mary Pat; Wright, Ann (December 2015). "A physiologist's view of homeostasis". Advances in Physiology Education. 39 (4): 259–266. doi:10.1152/advan.00107.2015. ISSN 1043-4046. PMC 4669363. PMID 26628646.
- Davies, PC; Rieper, E; Tuszynski, JA (January 2013). "Self-organization and entropy reduction in a living cell". Bio Systems. 111 (1): 1–10. doi:10.1016/j.biosystems.2012.10.005. PMC 3712629. PMID 23159919.
- Based on definition from: "Aquarena Wetlands Project glossary of terms". Texas State University at San Marcos. Archived from the original on 2004-06-08.
- Craig, Nancy (2014). Molecular Biology, Principles of Genome Function. ISBN 978-0-19-965857-2.
- Mosconi, Francesco; Julou, Thomas; Desprat, Nicolas; Sinha, Deepak Kumar; Allemand, Jean-François; Vincent Croquette; Bensimon, David (2008). "Some nonlinear challenges in biology". Nonlinearity. 21 (8): T131. Bibcode:2008Nonli..21..131M. doi:10.1088/0951-7715/21/8/T03. ISSN 0951-7715. S2CID 119808230.
- Howell, Elizabeth (8 December 2014). "How Did Life Become Complex, And Could It Happen Beyond Earth?". Astrobiology Magazine. Archived from the original on 17 August 2018. Retrieved 14 February 2018.
- ^ Pearce, Ben K.D.; Tupper, Andrew S.; Pudritz, Ralph E.; et al. (March 1, 2018). "Constraining the Time Interval for the Origin of Life on Earth". Astrobiology. 18 (3): 343–364. arXiv:1808.09460. Bibcode:2018AsBio..18..343P. doi:10.1089/ast.2017.1674. ISSN 1531-1074. PMID 29570409. S2CID 4419671.
- ^ Lindberg, David C. (2007). "Science before the Greeks". The beginnings of Western science: the European Scientific tradition in philosophical, religious, and institutional context (Second ed.). Chicago, Illinois: University of Chicago Press. pp. 1–20. ISBN 978-0-226-48205-7.
- ^ Grant, Edward (2007). "Ancient Egypt to Plato". A History of Natural Philosophy: From the Ancient World to the Nineteenth Century (First ed.). New York, New York: Cambridge University Press. pp. 1–26. ISBN 978-052-1-68957-1.
- Magner, Lois N. (2002). A History of the Life Sciences, Revised and Expanded. CRC Press. ISBN 978-0-203-91100-6. Archived from the original on 2015-03-24.
- Serafini, Anthony (2013). The Epic History of Biology. ISBN 978-1-4899-6327-7. Archived from the original on 15 April 2021. Retrieved 14 July 2015.
-
 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Theophrastus". Encyclopædia Britannica (11th ed.). Cambridge University Press.
One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Theophrastus". Encyclopædia Britannica (11th ed.). Cambridge University Press.
- Fahd, Toufic (1996). "Botany and agriculture". In Morelon, Régis; Rashed, Roshdi (eds.). Encyclopedia of the History of Arabic Science. Vol. 3. Routledge. p. 815. ISBN 978-0-415-12410-2.
- Magner, Lois N. (2002). A History of the Life Sciences, Revised and Expanded. CRC Press. pp. 133–44. ISBN 978-0-203-91100-6. Archived from the original on 2015-03-24.
- Sapp, Jan (2003). "7". Genesis: The Evolution of Biology. New York: Oxford University Press. ISBN 978-0-19-515618-8.
- Coleman, William (1977). Biology in the Nineteenth Century: Problems of Form, Function, and Transformation. New York: Cambridge University Press. ISBN 978-0-521-29293-1.
- Mayr, Ernst. The Growth of Biological Thought, chapter 4
- Mayr, Ernst. The Growth of Biological Thought, chapter 7
- Darwin 1909, p. 53
- Gould, Stephen Jay. The Structure of Evolutionary Theory. The Belknap Press of Harvard University Press: Cambridge, 2002. ISBN 0-674-00613-5. p. 187.
- Lamarck (1914)
- Mayr, Ernst. The Growth of Biological Thought, chapter 10: "Darwin's evidence for evolution and common descent"; and chapter 11: "The causation of evolution: natural selection"
- Larson, Edward J. (2006). "Ch. 3". Evolution: The Remarkable History of a Scientific Theory. Random House Publishing Group. ISBN 978-1-58836-538-5. Archived from the original on 2015-03-24.
- Henig (2000). Op. cit. pp. 134–138.
- ^ Miko, Ilona (2008). "Gregor Mendel's principles of inheritance form the cornerstone of modern genetics. So just what are they?". Nature Education. 1 (1): 134. Archived from the original on 2019-07-19. Retrieved 2021-05-13.
- Futuyma, Douglas J.; Kirkpatrick, Mark (2017). "Evolutionary Biology". Evolution (4th ed.). Sunderland, Mass.: Sinauer Associates. pp. 3–26.
- Noble, Ivan (2003-04-14). "Human genome finally complete". BBC News. Archived from the original on 2006-06-14. Retrieved 2006-07-22.
- ^ Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "The chemical context of life". Campbell Biology (11th ed.). New York: Pearson. pp. 28–43. ISBN 978-0134093413.
- ^ Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Water and carbon: The chemical basis of life". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 55–77. ISBN 978-0321976499.
- ^ Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "Carbon and the molecular diversity of life". Campbell Biology (11th ed.). New York: Pearson. pp. 56–65. ISBN 978-0134093413.
- Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Carbon and molecular diversity of life". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 56–65. ISBN 978-1464175121.
- ^ Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Protein structure and function". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 78–92. ISBN 978-0321976499.
- ^ Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "The structure and function of large biological molecules". Campbell Biology (11th ed.). New York: Pearson. pp. 66–92. ISBN 978-0134093413.
- ^ Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "An introduction to carbohydrate". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 107–118. ISBN 978-0321976499.
- ^ Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Lipids, membranes, and the first cells". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 119–141. ISBN 978-0321976499.
- ^ Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Nucleic acids and the RNA world". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 93–106. ISBN 978-0321976499.
- Mazzarello, P (May 1999). "A unifying concept: the history of cell theory". Nature Cell Biology. 1 (1): E13–15. doi:10.1038/8964. PMID 10559875. S2CID 7338204.
- Campbell NA, Williamson B, Heyden RJ (2006). Biology: Exploring Life. Boston: Pearson Prentice Hall. ISBN 9780132508827. Archived from the original on 2014-11-02. Retrieved 2021-05-13.
- Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "Membrane structure and function". Campbell Biology (11th ed.). New York: Pearson. pp. 126–142. ISBN 978-0134093413.
- Alberts B, Johnson A, Lewis J, et al. (2002). Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3. Archived from the original on 2017-12-20.
- Tom Herrmann; Sandeep Sharma (March 2, 2019). "Physiology, Membrane". StatPearls. PMID 30855799. Archived from the original on February 17, 2022. Retrieved May 14, 2021.
- Cell Movements and the Shaping of the Vertebrate Body Archived 2020-01-22 at the Wayback Machine in Chapter 21 of Molecular Biology of the Cell Archived 2017-09-27 at the Wayback Machine fourth edition, edited by Bruce Alberts (2002) published by Garland Science.
The Alberts text discusses how the "cellular building blocks" move to shape developing embryos. It is also common to describe small molecules such as amino acids as "molecular building blocks Archived 2020-01-22 at the Wayback Machine". - ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Cells: The working units of life". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 60–81. ISBN 978-1464175121.
- Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Energy and enzymes: An introduction to metabolism". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 171–188. ISBN 978-0321976499.
- Bailey, Regina. "Cellular Respiration". Archived from the original on 2012-05-05.
- ^ Lodish, Harvey; Berk, Arnold.; Kaiser, Chris A.; Krieger, Monty; Scott, Matthew P.; Bretscher, Anthony; Ploegh, Hidde; Matsudaira, Paul (2008). "Cellular energetics". Molecular Cell Biology (6th ed.). New York: W.H. Freeman and Company. pp. 479–532. ISBN 978-0716776017.
- "photosynthesis". Online Etymology Dictionary. Archived from the original on 2013-03-07. Retrieved 2013-05-23.
- φῶς. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- σύνθεσις. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- ^ Bryant DA, Frigaard NU (Nov 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends in Microbiology. 14 (11): 488–496. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
- Reece J, Urry L, Cain M, Wasserman S, Minorsky P, Jackson R (2011). Biology (International ed.). Upper Saddle River, N.J.: Pearson Education. pp. 235, 244. ISBN 978-0-321-73975-9.
This initial incorporation of carbon into organic compounds is known as carbon fixation.
- Neitzel, James; Rasband, Matthew. "Cell communication". Nature Education. Archived from the original on 29 September 2010. Retrieved 29 May 2021.
- ^ "Cell signaling". Nature Education. Archived from the original on 31 October 2010. Retrieved 29 May 2021.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Cell membranes and signaling". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 82–104. ISBN 978-1464175121.
- Martin EA, Hine R (2020). A dictionary of biology (6th ed.). Oxford: Oxford University Press. ISBN 9780199204625. OCLC 176818780.
- Griffiths AJ (2012). Introduction to genetic analysis (10th ed.). New York: W.H. Freeman and Co. ISBN 9781429229432. OCLC 698085201.
- "10.2 The Cell Cycle - Biology 2e | OpenStax". openstax.org. Archived from the original on 2020-11-29. Retrieved 2020-11-24.
- Freeman, Scott; Quillin, Kim; Allison, Lizabeth; Black, Michael; Podgorski, Greg; Taylor, Emily; Carmichael, Jeff (2017). "Meiosis". Biological Science (6th ed.). Hoboken, N.J.: Pearson. pp. 271–289. ISBN 978-0321976499.
- Casiraghi A, Suigo L, Valoti E, Straniero V (February 2020). "Targeting Bacterial Cell Division: A Binding Site-Centered Approach to the Most Promising Inhibitors of the Essential Protein FtsZ". Antibiotics. 9 (2): 69. doi:10.3390/antibiotics9020069. PMC 7167804. PMID 32046082.
- Griffiths, Anthony J.; Wessler, Susan R.; Carroll, Sean B.; Doebley, John (2015). "The genetics revolution". An Introduction to Genetic Analysis (11th ed.). Sunderland, Mass.: W.H. Freeman & Company. pp. 1–30. ISBN 978-1464109485.
- Griffiths, Anthony J.F.; Miller, Jeffrey H.; Suzuki, David T.; Lewontin, Richard C.; Gelbart, William M., eds. (2000). "Genetics and the Organism: Introduction". An Introduction to Genetic Analysis (7th ed.). New York: W. H. Freeman. ISBN 978-0-7167-3520-5.
- Hartl, D; Jones, E (2005). Genetics: Analysis of Genes and Genomes (6th ed.). Jones & Bartlett. ISBN 978-0-7637-1511-3.
- Rutgers: Mendelian Principles Archived 2021-05-14 at the Wayback Machine
- Miko, Ilona (2008), "Test crosses", Nature Education, 1 (1): 136, archived from the original on 2021-05-21, retrieved 2021-05-28
- Miko, Ilona (2008), "Thomas Hunt Morgan and sex linkage", Nature Education, 1 (1): 143, archived from the original on 2021-05-20, retrieved 2021-05-28
- "Pedigree". National Human Genome Research Institute. Archived from the original on 16 June 2021. Retrieved 28 May 2021.
A pedigree is a genetic representation of a family tree that diagrams the inheritance of a trait or disease though several generations. The pedigree shows the relationships between family members and indicates which individuals express or silently carry the trait in question.
- Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "Mendel and the gene idea". Campbell Biology (11th ed.). New York: Pearson. pp. 269–293. ISBN 978-0134093413.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "DNA and its role in heredity". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 172–193. ISBN 978-1464175121.
- Russell P (2001). iGenetics. New York: Benjamin Cummings. ISBN 0-8053-4553-1.
- Thanbichler, M; Wang, SC; Shapiro, L (October 2005). "The bacterial nucleoid: a highly organized and dynamic structure". Journal of Cellular Biochemistry. 96 (3): 506–21. doi:10.1002/jcb.20519. PMID 15988757. S2CID 25355087.
- "Genotype definition – Medical Dictionary definitions". Medterms.com. 2012-03-19. Archived from the original on 2013-09-21. Retrieved 2013-10-02.
- Crick FH (1958). "On protein synthesis". Symposia of the Society for Experimental Biology. 12: 138–63. PMID 13580867.
- Crick F (August 1970). "Central dogma of molecular biology". Nature. 227 (5258): 561–3. Bibcode:1970Natur.227..561C. doi:10.1038/227561a0. PMID 4913914. S2CID 4164029.
- "Central dogma reversed". Nature. 226 (5252): 1198–9. June 1970. Bibcode:1970Natur.226.1198.. doi:10.1038/2261198a0. PMID 5422595. S2CID 4184060.
- Lin, Yihan; Elowitz, Michael B. (2016). "Central Dogma Goes Digital". Molecular Cell. 61 (6): 791–792. doi:10.1016/j.molcel.2016.03.005. PMID 26990983. Archived from the original on 2021-10-03. Retrieved 2021-10-03.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Regulation of gene expression". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 215–233. ISBN 978-1464175121.
- Keene, Jack D.; Tenenbaum, Scott A. (2002). "Eukaryotic mRNPs may represent posttranscriptional operons". Molecular Cell. 9 (6): 1161–1167. doi:10.1016/s1097-2765(02)00559-2. PMID 12086614.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Genes, development, and evolution". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 273–298. ISBN 978-1464175121.
- Slack, J.M.W. (2013) Essential Developmental Biology. Wiley-Blackwell, Oxford.
- Slack, J.M.W. (2007). "Metaplasia and transdifferentiation: from pure biology to the clinic". Nature Reviews Molecular Cell Biology. 8 (5): 369–378. doi:10.1038/nrm2146. PMID 17377526. S2CID 3353748.
- Atala A, Lanza R (2012-12-31). Handbook of Stem Cells. Academic Press. p. 452. ISBN 978-0-12-385943-3. Archived from the original on 2021-04-12. Retrieved 2021-05-28.
- Yanes, Oscar; Clark, Julie; Wong, Diana M.; Patti, Gary J.; Sánchez-Ruiz, Antonio; Benton, H. Paul; Trauger, Sunia A.; Desponts, Caroline; Ding, Sheng; Siuzdak, Gary (June 2010). "Metabolic oxidation regulates embryonic stem cell differentiation". Nature Chemical Biology. 6 (6): 411–417. doi:10.1038/nchembio.364. ISSN 1552-4469. PMC 2873061. PMID 20436487.
- Carroll, Sean B. "The Origins of Form". Natural History. Archived from the original on 9 October 2018. Retrieved 9 October 2016.
Biologists could say, with confidence, that forms change, and that natural selection is an important force for change. Yet they could say nothing about how that change is accomplished. How bodies or body parts change, or how new structures arise, remained complete mysteries.
- Hall & Hallgrímsson 2007, pp. 4–6
- "Evolution Resources". Washington, D.C.: National Academies of Sciences, Engineering, and Medicine. 2016. Archived from the original on 2016-06-03.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Processes of evolution". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 299–324. ISBN 978-1464175121.
- Packard, Alpheus Spring (1901). Lamarck, the founder of Evolution: his life and work with translations of his writings on organic evolution. New York: Longmans, Green. ISBN 978-0-405-12562-1.
- ^ Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "Descent with modifications: A Darwinian view of life". Campbell Biology (11th ed.). New York: Pearson. pp. 466–483. ISBN 978-0134093413.
- "The Complete Works of Darwin Online – Biography". darwin-online.org.uk. Archived from the original on 2007-01-07. Retrieved 2006-12-15.
- Dobzhansky, T. (1973). "Nothing in biology makes sense except in the light of evolution". The American Biology Teacher. 35 (3): 125–29. CiteSeerX 10.1.1.525.3586. doi:10.2307/4444260. JSTOR 4444260. S2CID 207358177. - see also Nothing in Biology Makes Sense Except in the Light of Evolution
- Carroll, Joseph, ed. (2003). On the origin of species by means of natural selection. Peterborough, Ontario: Broadview. p. 15. ISBN 978-1-55111-337-1.
As Darwinian scholar Joseph Carroll of the University of Missouri–St. Louis puts it in his introduction to a modern reprint of Darwin's work: "The Origin of Species has special claims on our attention. It is one of the two or three most significant works of all time—one of those works that fundamentally and permanently alter our vision of the world ... It is argued with a singularly rigorous consistency but it is also eloquent, imaginatively evocative, and rhetorically compelling."
- Shermer p. 149.
- Lewontin, Richard C. (November 1970). "The Units of Selection" (PDF). Annual Review of Ecology and Systematics. 1: 1–18. doi:10.1146/annurev.es.01.110170.000245. ISSN 1545-2069. JSTOR 2096764. Archived (PDF) from the original on 2015-02-06.
- Darwin, Charles (1859). On the Origin of Species, John Murray.
- ^ Futuyma, Douglas J.; Kirkpatrick, Mark (2017). "Evolutionary biology". Evolution (4th ed.). Sunderland, Mass.: Sinauer Associates. pp. 3–26.
- Charlesworth, Brian; Charlesworth, Deborah (2009). "Darwin and genetics". Genetics. 183 (3): 757–766. doi:10.1534/genetics.109.109991. PMC 2778973. PMID 19933231.
- Futuyma, Douglas J.; Kirkpatrick, Mark (2017). "Mutation and variation". Evolution (4th ed.). Sunderland, Mass.: Sinauer Associates. pp. 79–101.
- Simpson, George Gaylord (1967). The Meaning of Evolution (Second ed.). Yale University Press. ISBN 978-0-300-00952-1.
- Masel, Joanna (October 25, 2011). "Genetic drift". Current Biology. 21 (20): R837 – R838. doi:10.1016/j.cub.2011.08.007. ISSN 0960-9822. PMID 22032182. S2CID 17619958.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Speciation". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 343–356. ISBN 978-1464175121.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Reconstructing and using phylogenies". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 325–342. ISBN 978-1464175121.
- Kitching, Ian J.; Forey, Peter L.; Williams, David M. (2001). "Cladistics". In Levin, Simon A. (ed.). Encyclopedia of Biodiversity (2nd ed.). Elsevier. pp. 33–45. doi:10.1016/B978-0-12-384719-5.00022-8. ISBN 9780123847201. Archived from the original on 29 August 2021. Retrieved 29 August 2021.)
- Futuyma, Douglas J.; Kirkpatrick, Mark (2017). "Phylogeny: The unity and diversity of life". Evolution (4th ed.). Sunderland, Mass.: Sinauer Associates. pp. 401–429.
- Woese, CR; Kandler, O; Wheelis, ML (June 1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4576–79. Bibcode:1990PNAS...87.4576W. doi:10.1073/pnas.87.12.4576. PMC 54159. PMID 2112744.
- McNeill, J; Barrie, FR; Buck, WR; Demoulin, V; Greuter, W; Hawksworth, DL; et al. (2012). International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. A.R.G. Gantner Verlag KG. ISBN 978-3-87429-425-6. Archived from the original on 2013-11-04. Recommendation 60F
- Silyn-Roberts, Heather (2000). Writing for Science and Engineering: Papers, Presentation. Oxford: Butterworth-Heinemann. p. 198. ISBN 978-0-7506-4636-9. Archived from the original on 2020-10-02. Retrieved 2020-08-24.
- Montévil, M; Mossio, M; Pocheville, A; Longo, G (October 2016). "Theoretical principles for biology: Variation". Progress in Biophysics and Molecular Biology. From the Century of the Genome to the Century of the Organism: New Theoretical Approaches. 122 (1): 36–50. doi:10.1016/j.pbiomolbio.2016.08.005. PMID 27530930. S2CID 3671068. Archived from the original on 2018-03-20.
- De Duve, Christian (2002). Life Evolving: Molecules, Mind, and Meaning. New York: Oxford University Press. p. 44. ISBN 978-0-19-515605-8.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "The history of life on Earth". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 357–376. ISBN 978-1464175121.
- "Stratigraphic Chart 2022" (PDF). International Stratigraphic Commission. February 2022. Archived (PDF) from the original on 2 April 2022. Retrieved 25 April 2022.
- Futuyma 2005
- Futuyma, DJ (2005). Evolution. Sinauer Associates. ISBN 978-0-87893-187-3. OCLC 57311264.
- Rosing, Minik T. (January 29, 1999). "C-Depleted Carbon Microparticles in >3700-Ma Sea-Floor Sedimentary Rocks from West Greenland". Science. 283 (5402): 674–676. Bibcode:1999Sci...283..674R. doi:10.1126/science.283.5402.674. ISSN 0036-8075. PMID 9924024.
- Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi; et al. (January 2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1): 25–28. Bibcode:2014NatGe...7...25O. doi:10.1038/ngeo2025. ISSN 1752-0894.
- Nisbet, Euan G.; Fowler, C.M.R. (December 7, 1999). "Archaean metabolic evolution of microbial mats". Proceedings of the Royal Society B. 266 (1436): 2375–2382. doi:10.1098/rspb.1999.0934. ISSN 0962-8452. PMC 1690475.
- Knoll, Andrew H.; Javaux, Emmanuelle J.; Hewitt, David; et al. (June 29, 2006). "Eukaryotic organisms in Proterozoic oceans". Philosophical Transactions of the Royal Society B. 361 (1470): 1023–1038. doi:10.1098/rstb.2006.1843. ISSN 0962-8436. PMC 1578724. PMID 16754612.
- Fedonkin, Mikhail A. (March 31, 2003). "The origin of the Metazoa in the light of the Proterozoic fossil record" (PDF). Paleontological Research. 7 (1): 9–41. doi:10.2517/prpsj.7.9. ISSN 1342-8144. S2CID 55178329. Archived from the original (PDF) on 2009-02-26. Retrieved 2008-09-02.
- Bonner, John Tyler (January 7, 1998). "The origins of multicellularity". Integrative Biology. 1 (1): 27–36. doi:10.1002/(SICI)1520-6602(1998)1:1<27::AID-INBI4>3.0.CO;2-6. ISSN 1757-9694.
- Strother, Paul K.; Battison, Leila; Brasier, Martin D.; et al. (May 26, 2011). "Earth's earliest non-marine eukaryotes". Nature. 473 (7348): 505–509. Bibcode:2011Natur.473..505S. doi:10.1038/nature09943. ISSN 0028-0836. PMID 21490597. S2CID 4418860.
- Beraldi-Campesi, Hugo (February 23, 2013). "Early life on land and the first terrestrial ecosystems". Ecological Processes. 2 (1): 1–17. doi:10.1186/2192-1709-2-1. ISSN 2192-1709.
- Algeo, Thomas J.; Scheckler, Stephen E. (January 29, 1998). "Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events". Philosophical Transactions of the Royal Society B. 353 (1365): 113–130. doi:10.1098/rstb.1998.0195. ISSN 0962-8436. PMC 1692181.
- Jun-Yuan, Chen; Oliveri, Paola; Chia-Wei, Li; et al. (April 25, 2000). "Precambrian animal diversity: Putative phosphatized embryos from the Doushantuo Formation of China". Proc. Natl. Acad. Sci. U.S.A. 97 (9): 4457–4462. Bibcode:2000PNAS...97.4457C. doi:10.1073/pnas.97.9.4457. ISSN 0027-8424. PMC 18256. PMID 10781044.
- D-G., Shu; H-L., Luo; Conway Morris, Simon; et al. (November 4, 1999). "Lower Cambrian vertebrates from south China" (PDF). Nature. 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965. ISSN 0028-0836. S2CID 4402854. Archived from the original (PDF) on 2009-02-26. Retrieved 2015-01-22.
- Hoyt, Donald F. (February 17, 1997). "Synapsid Reptiles". ZOO 138 Vertebrate Zoology (Lecture). Pomona, Calif.: California State Polytechnic University, Pomona. Archived from the original on 2009-05-20. Retrieved 2015-01-22.
- Barry, Patrick L. (January 28, 2002). Phillips, Tony (ed.). "The Great Dying". Science@NASA. Marshall Space Flight Center. Archived from the original on 2010-04-10. Retrieved 2015-01-22.
- Tanner, Lawrence H.; Lucas, Spencer G.; Chapman, Mary G. (March 2004). "Assessing the record and causes of Late Triassic extinctions" (PDF). Earth-Science Reviews. 65 (1–2): 103–139. Bibcode:2004ESRv...65..103T. doi:10.1016/S0012-8252(03)00082-5. Archived from the original (PDF) on 2007-10-25. Retrieved 2007-10-22.
- Benton 1997
- Fastovsky, David E.; Sheehan, Peter M. (March 2005). "The Extinction of the Dinosaurs in North America" (PDF). GSA Today. 15 (3): 4–10. doi:10.1130/1052-5173(2005)015<4:TEOTDI>2.0.CO;2. ISSN 1052-5173. Archived (PDF) from the original on 2019-03-22. Retrieved 2015-01-23.
- Roach, John (June 20, 2007). "Dinosaur Extinction Spurred Rise of Modern Mammals". National Geographic News. Washington, D.C.: National Geographic Society. Archived from the original on 2008-05-11. Retrieved 2020-02-21.
- Wible, John R.; Rougier, Guillermo W.; Novacek, Michael J.; et al. (June 21, 2007). "Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary". Nature. 447 (7147): 1003–1006. Bibcode:2007Natur.447.1003W. doi:10.1038/nature05854. ISSN 0028-0836. PMID 17581585. S2CID 4334424.
- Van Valkenburgh, Blaire (May 1, 1999). "Major Patterns in the History of Carnivorous Mammals". Annual Review of Earth and Planetary Sciences. 27: 463–493. Bibcode:1999AREPS..27..463V. doi:10.1146/annurev.earth.27.1.463. ISSN 1545-4495. Archived from the original on February 29, 2020. Retrieved May 15, 2021.
- Fredrickson JK, Zachara JM, Balkwill DL, Kennedy D, Li SM, Kostandarithes HM, Daly MJ, Romine MF, Brockman FJ (July 2004). "Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford site, Washington state". Applied and Environmental Microbiology. 70 (7): 4230–41. Bibcode:2004ApEnM..70.4230F. doi:10.1128/AEM.70.7.4230-4241.2004. PMC 444790. PMID 15240306.
- Dudek NK, Sun CL, Burstein D (2017). "Novel Microbial Diversity and Functional Potential in the Marine Mammal Oral Microbiome" (PDF). Current Biology. 27 (24): 3752–3762. doi:10.1016/j.cub.2017.10.040. PMID 29153320. S2CID 43864355. Archived (PDF) from the original on 2021-03-08. Retrieved 2021-05-14.
- Pace NR (May 2006). "Time for a change". Nature. 441 (7091): 289. Bibcode:2006Natur.441..289P. doi:10.1038/441289a. PMID 16710401. S2CID 4431143.
- Stoeckenius W (October 1981). "Walsby's square bacterium: fine structure of an orthogonal procaryote". Journal of Bacteriology. 148 (1): 352–60. doi:10.1128/JB.148.1.352-360.1981. PMC 216199. PMID 7287626.
- "Archaea Basic Biology". March 2018. Archived from the original on 2021-04-28. Retrieved 2021-05-14.
- Bang C, Schmitz RA (September 2015). "Archaea associated with human surfaces: not to be underestimated". FEMS Microbiology Reviews. 39 (5): 631–48. doi:10.1093/femsre/fuv010. PMID 25907112.
- Moissl-Eichinger C, Pausan M, Taffner J, Berg G, Bang C, Schmitz RA (January 2018). "Archaea Are Interactive Components of Complex Microbiomes". Trends in Microbiology. 26 (1): 70–85. doi:10.1016/j.tim.2017.07.004. PMID 28826642.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "The origin and diversification of eukaryotes". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 402–419. ISBN 978-1464175121.
- O'Malley, Maureen A.; Leger, Michelle M.; Wideman, Jeremy G.; Ruiz-Trillo, Iñaki (2019-02-18). "Concepts of the last eukaryotic common ancestor". Nature Ecology & Evolution. 3 (3). Springer Science and Business Media LLC: 338–344. doi:10.1038/s41559-019-0796-3. hdl:10261/201794. ISSN 2397-334X. PMID 30778187. S2CID 67790751.
- Taylor, F. J. R. 'M. (2003-11-01). "The collapse of the two-kingdom system, the rise of protistology and the founding of the International Society for Evolutionary Protistology (ISEP)". International Journal of Systematic and Evolutionary Microbiology. 53 (6). Microbiology Society: 1707–1714. doi:10.1099/ijs.0.02587-0. ISSN 1466-5026. PMID 14657097.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "The evolution of plants". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 420–449. ISBN 978-1464175121.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "The evolution and diversity of fungi". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 451–468. ISBN 978-1464175121.
- Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Animal origins and diversity". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 469–519. ISBN 978-1464175121.
- Wu KJ (15 April 2020). "There are more viruses than stars in the universe. Why do only some infect us? – More than a quadrillion quadrillion individual viruses exist on Earth, but most are not poised to hop into humans. Can we find the ones that are?". National Geographic Society. Archived from the original on 28 May 2020. Retrieved 18 May 2020.
- Koonin EV, Senkevich TG, Dolja VV (September 2006). "The ancient Virus World and evolution of cells". Biology Direct. 1 (1): 29. doi:10.1186/1745-6150-1-29. PMC 1594570. PMID 16984643.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Zimmer C (26 February 2021). "The Secret Life of a Coronavirus - An oily, 100-nanometer-wide bubble of genes has killed more than two million people and reshaped the world. Scientists don't quite know what to make of it". The New York Times. Archived from the original on 2021-12-28. Retrieved 28 February 2021.
- "Virus Taxonomy: 2019 Release". talk.ictvonline.org. International Committee on Taxonomy of Viruses. Archived from the original on 20 March 2020. Retrieved 25 April 2020.
- Lawrence CM, Menon S, Eilers BJ, Bothner B, Khayat R, Douglas T, Young MJ (May 2009). "Structural and functional studies of archaeal viruses". The Journal of Biological Chemistry. 284 (19): 12599–603. doi:10.1074/jbc.R800078200. PMC 2675988. PMID 19158076.
- Edwards RA, Rohwer F (June 2005). "Viral metagenomics". Nature Reviews. Microbiology. 3 (6): 504–10. doi:10.1038/nrmicro1163. PMID 15886693. S2CID 8059643.
- Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H (August 2003). "Phage as agents of lateral gene transfer". Current Opinion in Microbiology. 6 (4): 417–24. doi:10.1016/S1369-5274(03)00086-9. PMID 12941415.
- Rybicki EP (1990). "The classification of organisms at the edge of life, or problems with virus systematics". South African Journal of Science. 86: 182–86.
- Koonin EV, Starokadomskyy P (October 2016). "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question". Studies in History and Philosophy of Biological and Biomedical Sciences. 59: 125–34. doi:10.1016/j.shpsc.2016.02.016. PMC 5406846. PMID 26965225.
- Begon, M; Townsend, CR; Harper, JL (2006). Ecology: From individuals to ecosystems (4th ed.). Blackwell. ISBN 978-1-4051-1117-1.
- Habitats of the world. New York: Marshall Cavendish. 2004. p. 238. ISBN 978-0-7614-7523-1. Archived from the original on 2021-04-15. Retrieved 2020-08-24.
- Tansley (1934); Molles (1999), p. 482; Chapin et al. (2002), p. 380; Schulze et al. (2005); p. 400; Gurevitch et al. (2006), p. 522; Smith & Smith 2012, p. G-5
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "The distribution of Earth's ecological systems". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 845–863. ISBN 978-1464175121.
- Odum, Eugene P (1971). Fundamentals of Ecology (third ed.). New York: Saunders. ISBN 978-0-534-42066-6.
- Chapin III, F. Stuart; Matson, Pamela A.; Mooney, Harold A. (2002). "The ecosystem concept". Principles of Terrestrial Ecosystem Ecology. New York: Springer. p. 10. ISBN 978-0-387-95443-1.
- Planton, Serge, ed. (2013). "Annex III. Glossary: IPCC – Intergovernmental Panel on Climate Change" (PDF). IPCC Fifth Assessment Report. p. 1450. Archived from the original (PDF) on 2016-05-24. Retrieved 25 July 2016.
- Shepherd, Dr. J. Marshall; Shindell, Drew; O'Carroll, Cynthia M. (1 February 2005). "What's the Difference Between Weather and Climate?". NASA. Archived from the original on 22 September 2020. Retrieved 13 November 2015.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Populations". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 864–897. ISBN 978-1464175121.
- ^ Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Reece, Jane (2017). "Population ecology". Campbell Biology (11th ed.). New York: Pearson. pp. 1188–1211. ISBN 978-0134093413.
- "Population". Biology Online. Archived from the original on 13 April 2019. Retrieved 5 December 2012.
- "Definition of population (biology)". Oxford Dictionaries. Oxford University Press. Archived from the original on 4 March 2016. Retrieved 5 December 2012.
a community of animals, plants, or humans among whose members interbreeding occurs
- Hartl, Daniel (2007). Principles of Population Genetics. Sinauer Associates. p. 45. ISBN 978-0-87893-308-2.
- Chapman, Eric J.; Byron, Carrie J. (2018-01-01). "The flexible application of carrying capacity in ecology". Global Ecology and Conservation. 13: e00365. doi:10.1016/j.gecco.2017.e00365. ISSN 2351-9894.
- Odum, E. P.; Barrett, G. W. (2005). Fundamentals of Ecology (5th ed.). Brooks/Cole, a part of Cengage Learning. ISBN 978-0-534-42066-6. Archived from the original on 2011-08-20.
- Wootton, JT; Emmerson, M (2005). "Measurement of Interaction Strength in Nature". Annual Review of Ecology, Evolution, and Systematics. 36: 419–44. doi:10.1146/annurev.ecolsys.36.091704.175535. JSTOR 30033811.
- ^ Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Ecological and evolutionary consequences within and among species". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 882–897. ISBN 978-1464175121.
- Smith, AL (1997). Oxford dictionary of biochemistry and molecular biology. Oxford : Oxford University Press. p. 508. ISBN 978-0-19-854768-6.
Photosynthesis – the synthesis by organisms of organic chemical compounds, esp. carbohydrates, from carbon dioxide using energy obtained from light rather than the oxidation of chemical compounds.
- Edwards, Katrina. "Microbiology of a Sediment Pond and the Underlying Young, Cold, Hydrologically Active Ridge Flank". Woods Hole Oceanographic Institution.
- Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "Ecological communities". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 898–915. ISBN 978-1464175121.
- Riebeek, Holli (16 June 2011). "The Carbon Cycle". Earth Observatory. NASA. Archived from the original on 5 March 2016. Retrieved 5 April 2018.
- Hillis, David M.; Sadava, David; Hill, Richard W.; Price, Mary V. (2014). "The distribution of Earth's ecological systems". Principles of Life (2nd ed.). Sunderland, Mass.: Sinauer Associates. pp. 916–934. ISBN 978-1464175121.
- IPCC AR5 WG1 Summary for Policymakers 2013, p. 4: Warming of the climate system is unequivocal, and since the 1950s many of the observed changes are unprecedented over decades to millennia. The atmosphere and ocean have warmed, the amounts of snow and ice have diminished, sea level has risen, and the concentrations of greenhouse gases have increased; IPCC SR15 Ch1 2018, p. 54: Abundant empirical evidence of the unprecedented rate and global scale of impact of human influence on the Earth System (Steffen et al., 2016; Waters et al., 2016) has led many scientists to call for an acknowledgment that the Earth has entered a new geological epoch: the Anthropocene.
- EPA 2020: Carbon dioxide (76%), Methane (16%), Nitrous Oxide (6%).
- EPA 2020: Carbon dioxide enters the atmosphere through burning fossil fuels (coal, natural gas, and oil), solid waste, trees and other biological materials, and also as a result of certain chemical reactions (e.g., manufacture of cement). Fossil fuel use is the primary source of CO2. CO2 can also be emitted from direct human-induced impacts on forestry and other land use, such as through deforestation, land clearing for agriculture, and degradation of soils. Methane is emitted during the production and transport of coal, natural gas, and oil. Methane emissions also result from livestock and other agricultural practices and by the decay of organic waste in municipal solid waste landfills.
- Sahney, S.; Benton, M. J (2008). "Recovery from the most profound mass extinction of all time". Proceedings of the Royal Society B: Biological Sciences. 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
- Soulé, Michael E.; Wilcox, Bruce A. (1980). Conservation biology: an evolutionary-ecological perspective. Sunderland, Mass.: Sinauer Associates. ISBN 978-0-87893-800-1.
- Soulé, Michael E. (1986). "What is Conservation Biology?" (PDF). BioScience. 35 (11). American Institute of Biological Sciences: 727–34. doi:10.2307/1310054. JSTOR 1310054. Archived from the original (PDF) on 2019-04-12. Retrieved 2021-05-15.
- ^ Hunter, Malcolm L. (1996). Fundamentals of conservation biology. Oxford: Blackwell Science. ISBN 978-0-86542-371-8.
- ^ Meffe, Gary K.; Martha J. Groom (2006). Principles of conservation biology (3rd ed.). Sunderland, Mass.: Sinauer Associates. ISBN 978-0-87893-518-5.
- ^ Van Dyke, Fred (2008). Conservation biology: foundations, concepts, applications (2nd ed.). New York: Springer-Verlag. doi:10.1007/978-1-4020-6891-1. ISBN 9781402068904. OCLC 232001738. Archived from the original on 2020-07-27. Retrieved 2021-05-15.
- Sahney, S.; Benton, M. J.; Ferry, P. A. (2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land". Biology Letters. 6 (4): 544–7. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
- Koh, Lian Pin; Dunn, Robert R.; Sodhi, Navjot S.; Colwell, Robert K.; Proctor, Heather C.; Smith, Vincent S. (2004). "Species coextinctions and the biodiversity crisis". Science. 305 (5690): 1632–4. Bibcode:2004Sci...305.1632K. doi:10.1126/science.1101101. PMID 15361627. S2CID 30713492.
- Millennium Ecosystem Assessment (2005). Ecosystems and Human Well-being: Biodiversity Synthesis. World Resources Institute, Washington, D.C. Archived 2019-10-14 at the Wayback Machine
- Jackson, J. B. C. (2008). "Ecological extinction and evolution in the brave new ocean". Proceedings of the National Academy of Sciences. 105 (Suppl 1): 11458–65. Bibcode:2008PNAS..10511458J. doi:10.1073/pnas.0802812105. PMC 2556419. PMID 18695220.
- Soule, Michael E. (1986). Conservation Biology: The Science of Scarcity and Diversity. Sinauer Associates. p. 584. ISBN 978-0-87893-795-0.
Further reading
Further information: Bibliography of biology- Benton, Michael J. (1997). Vertebrate Palaeontology (2nd ed.). London: Chapman & Hall. ISBN 978-0-412-73800-5. OCLC 37378512.
- Darwin, Francis, ed. (1909). The foundations of The origin of species, a sketch written in 1842 (PDF). Cambridge: Printed at the University Press. LCCN 61057537. OCLC 1184581. Archived (PDF) from the original on 4 March 2016. Retrieved 27 November 2014.
- Hall, Brian K.; Hallgrímsson, Benedikt (6 December 2007). Strickberger's Evolution. Jones & Bartlett Publishers. ISBN 978-1-4496-4722-3.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular Biology of the Cell (4th ed.). Garland. ISBN 978-0-8153-3218-3. OCLC 145080076.
- Begon M, Townsend CR, Harper JL (2005). Ecology: From Individuals to Ecosystems (4th ed.). Blackwell Publishing Limited. ISBN 978-1-4051-1117-1. OCLC 57639896.
- Campbell N (2004). Biology (7th ed.). Benjamin-Cummings Publishing Company. ISBN 978-0-8053-7146-8. OCLC 71890442.
- Colinvaux P (1979). Why Big Fierce Animals are Rare: An Ecologist's Perspective (reissue ed.). Princeton University Press. ISBN 978-0-691-02364-9. OCLC 10081738.
- Mayr, Ernst (1982). The Growth of Biological Thought: Diversity, Evolution, and Inheritance. Harvard University Press. ISBN 978-0-674-36446-2. Archived from the original on 2015-10-03. Retrieved 2015-06-27.
- Hoagland M (2001). The Way Life Works (reprint ed.). Jones and Bartlett Publishers inc. ISBN 978-0-7637-1688-2. OCLC 223090105.
- Janovy, John (2004). On Becoming a Biologist (2nd ed.). Bison Books. ISBN 978-0-8032-7620-8. OCLC 55138571.
- Johnson, George B. (2005). Biology, Visualizing Life. Holt, Rinehart, and Winston. ISBN 978-0-03-016723-2. OCLC 36306648.
- Tobin, Allan; Dusheck, Jennie (2005). Asking About Life (3rd ed.). Belmont, Calif.: Wadsworth. ISBN 978-0-534-40653-0.
- IPCC (2013). Stocker, T. F.; Qin, D.; Plattner, G.-K.; Tignor, M.; et al. (eds.). Climate Change 2013: The Physical Science Basis (PDF). Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK & New York: Cambridge University Press. ISBN 978-1-107-05799-9..
- IPCC (2013). "Summary for Policymakers" (PDF). IPCC AR5 WG1 2013.
- IPCC (2018). Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; et al. (eds.). Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty (PDF). Intergovernmental Panel on Climate Change. Global Warming of 1.5 ºC —.
- Allen, M. R.; Dube, O. P.; Solecki, W.; Aragón-Durand, F.; et al. (2018). "Chapter 1: Framing and Context" (PDF). IPCC SR15 2018. pp. 49–91.
- US EPA (15 September 2020). "Overview of Greenhouse Gases". Retrieved 15 September 2020.
External links
- Template:Curlie
- OSU's Phylocode
- Biology Online – Wiki Dictionary
- MIT video lecture series on biology
- OneZoom Tree of Life
Journal links
- PLOS Biology A peer-reviewed, open-access journal published by the Public Library of Science
- Current Biology: General journal publishing original research from all areas of biology
- Biology Letters: A high-impact Royal Society journal publishing peer-reviewed biology papers of general interest
- Science: Internationally renowned AAAS science journal – see sections of the life sciences
- International Journal of Biological Sciences: A biological journal publishing significant peer-reviewed scientific papers
- Perspectives in Biology and Medicine: An interdisciplinary scholarly journal publishing essays of broad relevance
| History of biology (timeline) | |
|---|---|
| Fields, disciplines | |
| Theories, concepts | |
| Related | |
| Topics related to biology | |
|---|---|
| People and history | |
| Institutions, publications | |
| Terms and phrases | |
| Related disciplines | |
| Other |
|
| Branches of biology | |
|---|---|
| |
| See also | |
| Natural science | |
|---|---|
| Elements of nature | |||||
|---|---|---|---|---|---|
| Universe | |||||
| Earth | |||||
| Weather | |||||
| Natural environment | |||||
| Life | |||||
| See also | |||||
Categories:

