This is the current revision of this page, as edited by Discospinster (talk | contribs) at 19:47, 13 May 2023 (Reverted edits by 2600:1002:B057:B7F3:703A:C73C:3C47:2240 (talk) to last version by Graeme Bartlett). The present address (URL) is a permanent link to this version.
Revision as of 19:47, 13 May 2023 by Discospinster (talk | contribs) (Reverted edits by 2600:1002:B057:B7F3:703A:C73C:3C47:2240 (talk) to last version by Graeme Bartlett)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)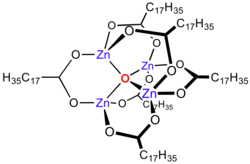
| |
| Names | |
|---|---|
| IUPAC name zinc octadecanoate | |
| Other names zinc distearate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.008.321 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C36H70O4Zn |
| Molar mass | 632.33 g·mol |
| Appearance | soft, white powder |
| Odor | slight, characteristic |
| Density | 1.095 g/cm, solid |
| Melting point | 120 to 130 °C (248 to 266 °F; 393 to 403 K) |
| Boiling point | decomposes |
| Solubility in water | insoluble |
| Solubility in Ethanol | insoluble |
| Solubility in ether | insoluble |
| Solubility in benzene | slightly soluble |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H335, H400, H413 |
| Precautionary statements | P261, P271, P273, P304+P340, P312, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 277 °C (531 °F; 550 K) |
| Autoignition temperature |
420 °C (788 °F; 693 K) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 15 mg/m (total) TWA 5 mg/m (resp) |
| REL (Recommended) | TWA 10 mg/m (total) TWA 5 mg/m (resp) |
| IDLH (Immediate danger) | N.D. |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Zinc stearate is a "zinc soap" that is widely used industrially. In this context, soap is used in its formal sense, a metal salt of a fatty acid: in this case stearic acid. It is a white solid that repels water. It is insoluble in polar solvents such as alcohol and ether but soluble in aromatic hydrocarbons (e.g., benzene) and chlorinated hydrocarbons when heated. It is the most powerful mold release agent among all metal soaps. It contains no electrolyte and has a hydrophobic effect. Its main application areas are the plastics and rubber industry, where it is used as a releasing agent and lubricant which can be easily incorporated.
Zinc carboxylates, e.g. basic zinc acetate, adopt complex formulas, and are not simply dicarboxylates of zinc. Instead the formula for most zinc carboxylates is Zn4O(O2CR)6, consisting of a Zn4O core with carboxylate ligands spanning the edges.
Applications
It is widely used as a release agent for the production of many kinds of objects: rubber, polyurethane, polyester processing system, powder metallurgy. These applications exploit its "non-stick" properties. In cosmetics, zinc stearate is a lubricant and thickening agent used to improve texture.
It is an "activator" for accelerated rubber sulfur vulcanization. As discovered in the early days of vulcanization, zinc has a beneficial effect on the reaction of the sulfur with the polyolefin. The stearate is a form of zinc that is highly soluble in the nonpolar medium of the polyolefins.
Being lipophilic, it functions as a phase transfer catalyst for the saponification of fats.
Niche uses
It is a component of some paints, imparting gloss. As a chief ingredient in "fanning powder", it is used by magicians performing card manipulation to decrease the friction between playing cards.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0676". National Institute for Occupational Safety and Health (NIOSH).
- "ZINC stearate". pubchem.ncbi.nlm.nih.gov.
- ^ David J. Anneken, Sabine Both, Ralf Christoph, Georg Fieg, Udo Steinberner, Alfred Westfechtel "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_245.pub2
- "Zinc Stearate | Cosmetics Info". cosmeticsinfo.org. Archived from the original on 2013-09-06.