This is the current revision of this page, as edited by Headbomb (talk | contribs) at 02:40, 3 August 2023 (Alter: journal. | Use this tool. Report bugs. | #UCB_Gadget). The present address (URL) is a permanent link to this version.
Revision as of 02:40, 3 August 2023 by Headbomb (talk | contribs) (Alter: journal. | Use this tool. Report bugs. | #UCB_Gadget)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) This article is about thymolphthalein. For other related dyes in the phthalein family, see Phthalein dye.
| |
| Names | |
|---|---|
| Preferred IUPAC name 3,3-Bis-2-benzofuran-1(3H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.300 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H30O4 |
| Molar mass | 430.544 g·mol |
| Appearance | White powder |
| Melting point | 248 to 252 °C (478 to 486 °F; 521 to 525 K) (decomposes) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H341, H350, H361 |
| Precautionary statements | P201, P202, P210, P233, P240, P241, P242, P243, P280, P281, P303+P361+P353, P308+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Thymolphthalein is a phthalein dye used as an acid–base (pH) indicator. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The molar extinction coefficient for the blue thymolphthalein dianion is 38,000 M cm at 595 nm.
| Thymolphthalein (pH indicator) | ||
| below pH 9.3 | above pH 10.5 | |
| 9.3 | ⇌ | 10.5 |
Thymolphthalein is also known to have use as a laxative and for disappearing ink.
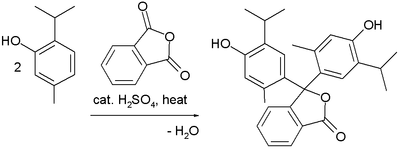
Preparation
Thymolphthalein can be synthesized from thymol and phthalic anhydride.
See also
References
- "Thymolphthalein". pubchem.ncbi.nlm.nih.gov.
- Hahn HH; Cheuk SF; Elfenbein S; Wood WB (April 1970). "Studies on the Pathogenesis of Fever: Xix. Localization of Pyrogen in Granulocytes". The Journal of Experimental Medicine. 131 (4): 701–9. doi:10.1084/jem.131.4.701. PMC 2138774. PMID 5430784.
- Hubacher, MH; Doernberg, S; Horner, A (1953). "Laxatives: chemical structure and potency of phthaleins and hydroxyanthraquinones". Journal of the American Pharmaceutical Association. 42 (1): 23–30. doi:10.1002/jps.3030420108. PMID 13034620.
- Katz, David A. (1982). "Disappearing Ink" (PDF). www.chymist.com. Retrieved August 14, 2017.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |