This is the current revision of this page, as edited by Citation bot (talk | contribs) at 22:01, 2 December 2023 (Add: doi-access. | Use this bot. Report bugs. | Suggested by Headbomb | #UCB_toolbar). The present address (URL) is a permanent link to this version.
Revision as of 22:01, 2 December 2023 by Citation bot (talk | contribs) (Add: doi-access. | Use this bot. Report bugs. | Suggested by Headbomb | #UCB_toolbar)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)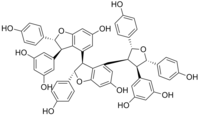
| |
| Names | |
|---|---|
| Preferred IUPAC name (2S,2′R,3S,3′R)-3′-(3,5-Dihydroxyphenyl)-4--2,2′-bis(hydroxyphenyl)-2,2′,3,3′-tetrahydro-6,6′-diol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C56H44O13 |
| Molar mass | 924.94 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Carasinol B is a stilbenoid tetramer found in Caragana sinica (Chinese : Jin Que-gen).
Acid-catalyzed epimerization of kobophenol A to carasinol B can be performed in vitro.
References
- Shu Na; Zhou Hong; Hu Changqi (2006). "Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method". Biological & Pharmaceutical Bulletin. 29 (4): 608–612. doi:10.1248/bpb.29.608. PMID 16595888.
- Kejun Cheng; Gaolin Liang; Changqi Hu (2008). "Acid-catalyzed Epimerization of Kobophenol A to Carasinol B". Molecules. 13 (4): 938–942. doi:10.3390/molecules13040938. PMC 6245474. PMID 18463595.
| Oligostilbenoids and their glycosides | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers |
| ||||||||||||
| Trimers | |||||||||||||
| Tetramers: |
| ||||||||||||
| Higher polymers (five units or more) |
| ||||||||||||
| Oligomeric forms of resveratrol |
| ||||||||||||
| Glycosides or conjugates |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |