This is the current revision of this page, as edited by Whywhenwhohow (talk | contribs) at 06:01, 31 December 2023 (→top: infobox). The present address (URL) is a permanent link to this version.
Revision as of 06:01, 31 December 2023 by Whywhenwhohow (talk | contribs) (→top: infobox)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Chemical compound Pharmaceutical compound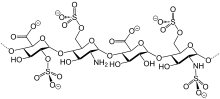 | |
| Clinical data | |
|---|---|
| Trade names | Fraxiparin(e), Fraxodi, others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Subcutaneous injection (except for haemodialysis) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89% (SC dose) |
| Elimination half-life | 3.7 hours (SC dose) |
| Excretion | clearance 21.4mL/min (+/- 7) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.029.698 |
| Chemical and physical data | |
| Molar mass | 4300 g/mol |
| (what is this?) (verify) | |
Nadroparin (trade names Fraxiparin, Fraxodi, among others) is an anticoagulant belonging to a class of drugs called low molecular weight heparins (LMWHs). Nadroparin was developed by Sanofi-Synthélabo.
Nadroparin is used in general and orthopedic surgery to prevent thromboembolic disorders (deep vein thrombosis and pulmonary embolism), and as treatment for deep vein thrombosis. It is also used to prevent clotting during hemodialysis, and for treatment of unstable angina and non-Q wave myocardial infarction.
For the treatment and prevention of DVT, the drug is administered as a subcutaneous injection (under the skin), usually around the abdomen. It is on the World Health Organization's List of Essential Medicines.
References
- "Fraxiparine PRODUCT MONOGRAPH (Canada)" (PDF). Aspripharma.com. Retrieved 2 April 2019.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
External links
- "NCI Drug Dictionary". National Cancer Institute. 2 February 2011. Retrieved 2 April 2019.
- Shafiq N, Malhotra S, Pandhi P, Sharma N, Bhalla A, Grover A (2006). "A randomized controlled clinical trial to evaluate the efficacy, safety, cost-effectiveness and effect on PAI-1 levels of the three low-molecular-weight heparins--enoxaparin, nadroparin and dalteparin. The ESCAPe-END study". Pharmacology. 78 (3): 136–43. doi:10.1159/000096484. PMID 17057417. S2CID 95878036.
| Antithrombotics (thrombolytics, anticoagulants and antiplatelet drugs) (B01) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatelet drugs |
| ||||||||||||||
| Anticoagulants |
| ||||||||||||||
| Thrombolytic drugs/ fibrinolytics | |||||||||||||||
| Non-medicinal | |||||||||||||||
| |||||||||||||||
This drug article relating to the blood and blood forming organs is a stub. You can help Misplaced Pages by expanding it. |