This is the current revision of this page, as edited by Newspaceplan (talk | contribs) at 03:54, 5 February 2024. The present address (URL) is a permanent link to this version.
Revision as of 03:54, 5 February 2024 by Newspaceplan (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |

| |
| Names | |
|---|---|
| IUPAC name Iron(II) oxalate | |
| Other names
Iron oxalate Ferrous oxalate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.007.472 |
| EC Number |
|
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | FeC2O4 (anhydrous) FeC2O4 · 2 H2O (dihydrate) |
| Molar mass | 143.86 g/mol (anhydrous) 179.89 g/mol (dihydrate) |
| Appearance | yellow powder |
| Odor | odorless |
| Density | 2.28 g/cm |
| Melting point | dihydrate: 150–160 °C (302–320 °F; 423–433 K) (decomposes) |
| Solubility in water | dihydrate: 0.097 g/100ml (25 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312 |
| Precautionary statements | P280 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ferrous oxalate (iron(II) oxalate) are inorganic compound with the formula FeC2O4(H2O)x where x is 0 or 2. These are orange compounds, poorly soluble in water.
Structure and reactions
Like other iron oxalates, ferrous oxalates feature octahedral Fe centers. The dihydrate FeC2O4(H2O)x is a coordination polymer, consisting of chains of oxalate-bridged ferrous centers, each with two aquo ligands.
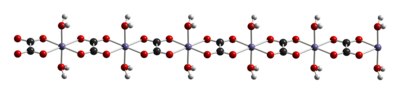
When heated to 120 °C, the dihydrate dehydrates, and the anhydrous ferrous oxalate decomposes near 190 °C. The products of thermal decomposition is a mixture of iron oxides and pyrophoric iron metal, as well as released carbon dioxide, carbon monoxide, and water.
Ferrous oxalates are precursors to iron phosphates, which are of value in batteries.
Natural occurrence
Anhydrous iron(II) oxalate is unknown among minerals as of 2020. However, the dihydrate is known as humboldtine. A related, though much more complex mineral is stepanovite,
Na ·3H2O - an example of trioxalatoferrate(III).
See also
References
- "Iron(II) oxalate dihydrate".
- ^ Sigma-Aldrich Co., Iron(II) oxalate dihydrate. Retrieved on 2014-05-03.
- Echigo, Takuya; Kimata, Mitsuyoshi (2008). "Single-crystal X-ray diffraction and spectroscopic studies on humboldtine and lindbergite: weak Jahn–Teller effect of Fe ion". Physics and Chemistry of Minerals. 35 (8): 467–475. Bibcode:2008PCM....35..467E. doi:10.1007/s00269-008-0241-7. S2CID 98739882.
- Mu, Jacob; Perlmutter, D.D. (1981). "Thermal decomposition of carbonates, carboxylates, oxalates, acetates, formates, and hydroxides". Thermochimica Acta. 49 (2–3): 207–218. doi:10.1016/0040-6031(81)80175-x.
- Hermanek, Martin; Zboril, Radek; Mashlan, Miroslav; Machala, Libor; Schneeweiss, Oldrich (2006). "Thermal Behaviour of Iron(II) Oxalate Dihydrate in the Atmosphere of Its Conversion Gases". J. Mater. Chem. 16 (13): 1273–1280. doi:10.1039/b514565a.
- Ellis, B. L.; Makahnouk, W. R. M.; Makimura, Y.; Toghill, K.; Nazar, L. F. (2007). "A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries". Nature Materials. 6 (10): 749–753. Bibcode:2007NatMa...6..749E. doi:10.1038/nmat2007. PMID 17828278.
- "Humboldtine".
- ^ "List of Minerals". 21 March 2011.
- "Stepanovite".
| Iron compounds | |||
|---|---|---|---|
| Fe(−II) | |||
| Fe(0) | |||
| Fe(I) |
| ||
| Fe(0,II) | |||
| Fe(II) |
| ||
| Fe(0,III) | |||
| Fe(II,III) | |||
| Fe(III) |
| ||
| Fe(IV) | |||
| Fe(VI) | |||
| Purported | |||
| sort | |||
| Compounds of the oxalate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||