This is an old revision of this page, as edited by 69.142.192.250 (talk) at 03:26, 6 April 2007 (→History). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:26, 6 April 2007 by 69.142.192.250 (talk) (→History)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. Please help improve this article by introducing more precise citations. (Learn how and when to remove this message) |
Chemistry (from the Egyptian kēme (chem), meaning earth) is the science that studies matter at the atomic to macromolecular scale, the reactions, transformations and aggregations of matter, as well as the energy and entropy released or absorbed during these processes. In short, chemistry studies molecules, crystals, and metals and is concerned with the composition and statistical properties of such structures, as well as their transformations and interactions to become materials encountered in everyday life. According to modern chemistry, the physical properties of materials are generally determined by their structure at the molecular or atomic scale, which is itself defined by interatomic electromagnetic forces, and laws of quantum mechanics and thermodynamics. Robert Boyle (1661), Antoine Lavoisier (1787), and John Dalton (1808) can be considered the three fathers of modern chemistry, although other scientists have played a vastly important role, such as Dmitri Mendeleyev, Friedrich Woehler, and Hermann Staudinger, to name but a few.
Text cannot be found
Etymology
Main article: Chemistry (etymology)The word chemistry comes from the earlier study of alchemy, which is basically the quest to make gold from earthen starting materials. As to the origin of the word "alchemy" the question is a debatable one; it certainly has Greek origins, and some, following E. Wallis Budge, have also asserted Egyptian origins. Alchemy, generally, derives from the old French alkemie and the Arabic al-kimia - "the art of transformation". The Arabs borrowed the word "kimia" from the Greeks when they conquered Alexandria in the year 642 AD. A tentative outline is as follows:
- Egyptian alchemy , formulate early "element" theories such as the Ogdoad.
- Greek alchemy , the Greek king Alexander the Great conquers Egypt and founds Alexandria, having the world's largest library, where scholars and "wise" men gather to study.
- Arabian alchemy , the Arabs take over Alexandria; Jabir is the main chemist
- European alchemy , Pseudo-Geber builds on Arabic chemistry
- Chemistry , Boyle writes his classic chemistry text The Sceptical Chymist
- Chemistry , Lavoisier writes his classic Elements of Chemistry
- Chemistry , Dalton publishes his Atomic Theory
Thus, an alchemist was called a 'chemist' in popular speech, and later the suffix "-ry" was added to this to describe the art of the chemist as "chemistry".
Definitions
In retrospect, the definition of chemistry seems to invariably change per decade, as new discoveries and theories add to the functionality of the science. Shown below, for example, are some of the standard definitions used by various noted chemists:
- Alchemy (330) – the study of the composition of waters, movement, growth, embodying and disembodying, drawing the spirits from bodies and bonding the spirits within bodies (Zosimos).
- Chymistry (1661) – the subject of the material principles of mixt bodies (Boyle).
- Chymistry (1663) – a scientifick art, by which one learns to dissolve bodies, and draw from them the different substances on their composition, and how to unite them again, and exalt them to an higher perfection (Glaser).
- Chemistry (1730) – the art of resolving mixt, compound, or aggregate bodies into their principles; and of composing such bodies from those principles (Stahl).
- Chemistry (1837) – the science concerned with the laws and effects of molecular forces (Dumas).
- Chemistry (1947) – the science of substances: their structure, their properties, and the reactions that change them into other substances (Pauling).
- Chemistry (1998) – the study of matter and the changes it undergoes (Chang).
Subdisciplines

Chemistry is typically divided into several major sub-disciplines. There are also several main cross-disciplinary and more specialized fields of chemistry.
- Analytical chemistry is the analysis of material samples to gain an understanding of their chemical composition and structure. Analytical chemistry incorporates standardized experimental methods in chemistry. These methods may be used in all subdisciplines of chemistry, excluding purely theoretical chemistry.
- Biochemistry is the study of the chemicals, chemical reactions and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics.
- Inorganic chemistry is the study of the properties and reactions of inorganic compounds. The distinction between organic and inorganic disciplines is not absolute and there is much overlap, most importantly in the sub-discipline of organometallic chemistry.
- Organic chemistry is the study of the structure, properties, composition, mechanisms, and reactions of organic compounds. An organic compound is defined as any compound based on a carbon skeleton.
- Physical chemistry is the study of the physical and fundamental basis of chemical systems and processes. In particular, the energetics and dynamics of such systems and processes are of interest to physical chemists. Important areas of study include chemical thermodynamics, chemical kinetics, electrochemistry, statistical mechanics, and spectroscopy. Physical chemistry has large overlap with molecular physics. Physical chemistry involves the use of calculus in deriving equations. It is usually associated with quantum chemistry and theoretical chemistry. Physical chemistry is a distinct discipline from chemical physics.
- Theoretical chemistry is the study of chemistry via fundamental theoretical reasoning (usually within mathematics or physics). In particular the application of quantum mechanics to chemistry is called quantum chemistry. Since the end of the Second World War, the development of computers has allowed a systematic development of computational chemistry, which is the art of developing and applying computer programs for solving chemical problems. Theoretical chemistry has large overlap with (theoretical and experimental) condensed matter physics and molecular physics. Essentially from reductionism theoretical chemistry is just physics, just like fundamental biology is just chemistry and physics.
- Nuclear chemistry is the study of how subatomic particles come together and make nuclei. Modern Transmutation is a large component of nuclear chemistry, and the table of nuclides is an important result and tool for this field.
- Pure Chemistry - The study of chemistry for chemistry's sake.
- Applied Chemistry - The study of chemistry directed at a goal possibly for money or military benefits.
Other fields include Astrochemistry, Atmospheric chemistry, Chemical Engineering, Chemo-informatics, Electrochemistry, Environmental chemistry, Flow chemistry, Geochemistry, Green chemistry, History of chemistry, Materials science, Medicinal chemistry, Molecular Biology, Molecular genetics, Nanotechnology, Organometallic chemistry, Petrochemistry, Pharmacology, Photochemistry, Phytochemistry, Polymer chemistry, Solid-state chemistry, Sonochemistry, Supramolecular chemistry, Surface chemistry, Immunochemistry and Thermochemistry.
The nature and classifications of matter

Atoms
Main article: AtomAn atom is a collection of matter consisting of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus. The Atom is also the smallest portion into which an element can be divided and still retain its properties, made up of a dense, positively charged nucleus surrounded by a system of electrons.
Elements
Main article: Chemical elementAn element is a class of atoms which have the same number of protons in the nucleus. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium.
The most convenient presentation of the chemical elements is in the periodic table of the chemical elements, which groups elements by atomic number. Due to its ingenious arrangement, groups, or columns, and periods, or rows, of elements in the table either share several chemical properties, or follow a certain trend in characteristics such as atomic radius, electronegativity, etc. Lists of the elements by name, by symbol, and by atomic number are also available. In addition, several isotopes of an element may exist.
Compounds
Main article: Chemical compoundA compound is a substance with a fixed ratio of chemical elements which determines the composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen between the hydrogens, and an angle of 104.5° between them. Compounds are formed and interconverted by chemical reactions.
Substance
Main article: Chemical substanceA chemical substance is a general term that can be an element, compound or a mixture of compounds, elements or compounds and elements. Most of the matter we encounter in our daily life are one or another kind of mixtures, e.g. air, alloys, biomass etc.
Molecules
Main article: MoleculeA molecule is the smallest indivisible portion of a pure compound or element that retains a set of unique chemical properties.
Ions and Salts
Main article: IonAn ion is a charged species, or an atom or a molecule that has lost or gained one or more electrons. Positively charged cations (e.g. sodium cation Na) and negatively charged anions (e.g. chloride Cl) can form neutral salts (e.g. sodium chloride NaCl). Examples of polyatomic ions that do not split up during acid-base reactions are hydroxide (OH) and phosphate (PO4).
States of matter
Main article: Phase (matter)In addition to the specific chemical properties that distinguish different chemical classifications chemicals can exist in several phases. For the most part, the chemical classifications are independent of these bulk phase classifications; however, some more exotic phases are incompatible with certain chemical properties. A phase is a set of states of a chemical system that have similar bulk structural properties, over a range of conditions, such as pressure or temperature. Physical properties, such as density and refractive index tend to fall within values characteristic of the phase. The phase of matter is defined by the phase transition, which is when energy put into or taken out of the system goes into rearranging the structure of the system, instead of changing the bulk conditions.
Sometimes the distinction between phases can be continuous instead of having a discrete boundary, in this case the matter is considered to be in a supercritical state. When three states meet based on the conditions, it is known as a triple point and since this is invariant, it is a convenient way to define a set of conditions.
The most familiar examples of phases are solids, liquids, and gases. Less familiar phases include plasmas, Bose-Einstein condensates and fermionic condensates and the paramagnetic and ferromagnetic phases of magnetic materials. Even the familiar ice has many different phases, depending on the pressure and temperature of the system. While most familiar phases deal with three-dimensional systems, it is also possible to define analogs in two-dimensional systems, which has received attention for its relevance to systems in biology.
Fundamental concepts and theories
Nomenclature
Main article: IUPAC nomenclatureNomenclature refers to the system for naming chemical compounds. There are well-defined systems in place for naming chemical species. Organic compounds are named according to the organic nomenclature system. Inorganic compounds are named according to the inorganic nomenclature system. Nomenclature is a critical part of the language of chemistry and the IUPAC system of chemical nomenclature used today allows chemists to specify by name specific compounds amongst the infinite variety of possible chemicals.
Chemical reactions
Main article: Chemical reactionA Chemical reaction is a process that results in the interconversion of chemical substances. Such reactions can result in molecules combining to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of atoms within or across molecules. Chemical reactions usually involve the making or breaking of chemical bonds. For example, substances that react with oxygen to produce other substances are said to undergo oxidation; similarly a group of substances called acids or alkalis can react with one another to neutralize each other's effect, a phenomenon known as neutralization. Substances can also be dissociated or synthesized from other substances by various different chemical processes.
A stricter definition exists that states "a Chemical Reaction is a process that results in the interconversion of chemical species". Under this definition, a chemical reaction may be an elementary reaction or a stepwise reaction. An additional caveat is made, in that this definition includes cases where the interconversion of conformers is experimentally observable. Such detectable chemical reactions normally involve sets of molecular entities as indicated by this definition, but it is often conceptually convenient to use the term also for changes involving single molecular entities (i.e. 'microscopic chemical events').
Chemical laws
Main article: Chemical lawThe most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction. Modern physics shows that it is actually energy that is conserved, and that energy and mass are related; a concept which becomes important in nuclear chemistry. Conservation of energy leads to the important concepts of equilibrium, thermodynamics, and kinetics.
Further laws of chemistry elaborate on the law of conservation of mass. Joseph Proust's law of definite composition says that pure chemicals are composed of elements in a definite formulation; we now know that the structural arrangement of these elements is also important.
Dalton's law of multiple proportions says that these chemicals will present themselves in proportions that are small whole numbers (i.e. 1:2 O:H in water); although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction. Such compounds are known as non-stoichiometric compounds.
Bonding
Main article: Chemical bond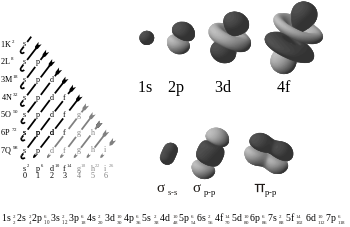
A chemical bond is the multipole balance between the positive charges in the nuclei and the negative charges oscillating about them. More than simple attraction and repulsion, the energies and distributions characterize the availability of an electron to bond to another atom. These potentials create the interactions which holds together atoms in molecules or crystals. In many simple compounds, Valence Bond Theory, the Valence Shell Electron Pair Repulsion model (VSEPR), and the concept of oxidation number can be used to predict molecular structure and composition. Similarly, theories from classical physics can be used to predict many ionic structures. With more complicated compounds, such as metal complexes, valence bond theory fails and alternative approaches, primarily based on principles of quantum chemistry such as the molecular orbital theory, are necessary. See diagram on electronic orbitals.
Quantum chemistry
Main article: Quantum chemistryQuantum chemistry mathematically describes the fundamental behavior of matter at the molecular scale. It is, in principle, possible to describe all chemical systems using this theory. In practice, only the simplest chemical systems may realistically be investigated in purely quantum mechanical terms, and approximations must be made for most practical purposes (e.g., Hartree-Fock, post Hartree-Fock or Density functional theory, see computational chemistry for more details). Hence a detailed understanding of quantum mechanics is not necessary for most chemistry, as the important implications of the theory (principally the orbital approximation) can be understood and applied in simpler terms.
In quantum mechanics (several applications in computational chemistry and quantum chemistry), the Hamiltonian, or the physical state, of a particle can be expressed as the sum of two operators, one corresponding to kinetic energy and the other to potential energy. The Hamiltonian in the Schrödinger wave equation used in quantum chemistry does not contain terms for the spin of the electron.
Solutions of the Schrödinger equation for the hydrogen atom gives the form of the wave function for atomic orbitals, and the relative energy of say the 1s,2s,2p and 3s orbitals. The orbital approximation can be used to understand the other atoms e.g. helium, lithium and carbon.
Chemical industry
Main article: chemical industryThe chemical industry represents an important economic activity. The global top 50 chemical producers in 2004 had sales of 587 billion US dollars with a profit margin of 8.1% and research and development spending of 2.1% of total chemical sales.
See also
Lists
- Common chemicals - Where to find common chemical components
- List of basic chemistry topics
- List of chemistry topics
- List of chemists
- List of compounds
- List of important publications in chemistry
- Periodic Table of the Elements
- Timeline of chemistry
- Unsolved problems in chemistry
Related topics
- Alchemy
- Biochemistry
- Chemical engineering
- History of chemistry
- Linus Pauling
- Registration, Evaluation and Authorisation of Chemicals - A proposed European Union regulation
- Perfection ("Perfection in physics and chemistry")
References
- See: Chemistry (etymology) for possible origins of this word.
- Mi Gyung, Kim (2003). Affinity, That Elusive Dream - A Genealogy of the Chemical Revolution. MIT Press. ISBN 0-262-11273-6.
- Strathern, P. (2000). Mendeleyev’s Dream – the Quest for the Elements. New York: Berkley Books.
- Boyle, Robert (1661). The Sceptical Chymist. New York: Dover Publications, Inc. (reprint). ISBN 0486428257.
- Glaser, Christopher (1663). Traite de la chymie. Paris. as found in: Kim, Mi Gyung (2003). Affinity, That Elusive Dream - A Geanealogy of the Chemical Revolution. The MIT Press. ISBN 0-262-11273-6.
- Stahl, George, E. (1730). Philosophical Principles of Universal Chemistry. London.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Dumas, J. B. (1837). 'Affinite' (lecture notes), vii, pg 4. “Statique chimique”, Paris: Academie des Sciences
- Pauling, Linus (1947). General Chemistry. Dover Publications, Inc. ISBN 0486656225.
- Chang, Raymond (1998). Chemistry, 6th Ed. New York: McGraw Hill. ISBN 0-07-115221-0.
- Gold Book Link
- "Top 50 Chemical Producers". Chemical & Engineering News. 83 (29): 20–23. July 18, 2005.
{{cite journal}}: Check date values in:|date=(help)
Further reading
Popular reading
- Atkins, P.W. Galileo's Finger (Oxford University Press) ISBN 0198609418
- Atkins, P.W. Atkins' Molecules (Cambridge University Press) ISBN 0521823978
- Stwertka, A. A Guide to the Elements (Oxford University Press) ISBN 0195150279
Introductory undergraduate text books
- Chang, Raymond. Chemistry 6th ed. Boston: James M. Smith, 1998. ISBN 0-07-115221-0.
- Pauling, L. The Nature of the chemical bond (Cornell University Press) ISBN 0-8014-0333-2
- Pauling, L., and Wilson, E. B. Introduction to Quantum Mechanics with Applications to Chemistry (Dover Publications) ISBN 0-486-64871-0
- Atkins, P.W., Overton, T., Rourke, J., Weller, M. and Armstrong, F. Shriver and Atkins inorganic chemistry (4th edition) 2006 (Oxford University Press) ISBN 0-19-926463-5
- Clayden, J., Greeves, N., Warren, S., Wothers, P. Organic Chemistry 2000 (Oxford University Press) ISBN 0-19-850346-6
- Voet and Voet Biochemistry (Wiley) ISBN 0-471-58651-X
Advanced Undergraduate-level or Graduate text books
- Atkins, P.W. Physical Chemistry (Oxford University Press) ISBN 0-19-879285-9
- Atkins, P.W. et al. Molecular Quantum Mechanics (Oxford University Press)
- McWeeny, R. Coulson's Valence (Oxford Science Publications) ISBN 0-19-855144-4
- Stephenson, G. Mathematical Methods for Science Students (Longman)ISBN 0-582-44416-0
- Smart and Moore Solid State Chemistry: An Introduction (Chapman and Hall) ISBN 0-412-40040-5
Professional societies
- American Chemical Society
- Chemical Institute of Canada
- Chemical Society of Peru
- International Union of Pure and Applied Chemistry
- Royal Australian Chemical Institute
- Royal Society of Chemistry
- Society of Chemical Industry
- World Association of Theoretical and Computational Chemists
External links
- International Union of Pure and Applied Chemistry
- IUPAC Nomenclature Home Page, see especially the "Gold Book" containing definitions of standard chemical terms
- Interactive Mind Map of Chemistry
For a full list of external links and suppliers see Misplaced Pages:Chemical sources
| Natural science | |
|---|---|
Template:Link FA Template:Link FA ru-sib:Химия
Categories: