This is the current revision of this page, as edited by 85.238.128.227 (talk) at 15:17, 23 February 2024 (→Reactions). The present address (URL) is a permanent link to this version.
Revision as of 15:17, 23 February 2024 by 85.238.128.227 (talk) (→Reactions)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
| Names | |
|---|---|
| Preferred IUPAC name 1,2λ-Oxathiolane-2,2-dione | |
| Other names γ-Propane sultone; 1,2-Oxathiolane, 2,2-dioxide; 3-Hydroxyl-1-propane sulfonic acid sulfone; 1-Propane sulfonic acid-3-hydroxyl-γ-sultone; Oxathiolane 2,2-dioxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.017 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H6O3S |
| Molar mass | 122.14 g·mol |
| Appearance | White crystalline solid; colorless liquid above 31 °C |
| Density | 1.392 g/cm at 40 °C |
| Melting point | 31 °C (88 °F; 304 K) |
| Boiling point | 112 °C (234 °F; 385 K) at 1.4 mm Hg |
| Solubility in water | 10% (20°C) |
| Hazards | |
| Flash point | 158 °C (316 °F; 431 K) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | none |
| REL (Recommended) | Ca |
| IDLH (Immediate danger) | Ca |
| Safety data sheet (SDS) | NIH.gov |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
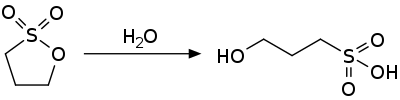
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones. It is a readily melting colorless solid.
Synthesis
It may be prepared by the acid catalyzed reaction of allyl alcohol and sodium bisulfite.
Reactions
1,3-propane sultone is an activated ester and is susceptible to nucleophilic attack. It hydrolyzes to the 3-hydroxypropylsulfonic acid.
It has been used in the synthesis of specialist surfactants, such as CHAPS detergent.
Safety
Typical of activated esters, 1,3-propane sultone is an alkylating agent. 1,3-Propane sultone is toxic, carcinogenic, mutagenic, and teratogenic.
See also
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0525". National Institute for Occupational Safety and Health (NIOSH).
- R. J. Cremlyn (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN 0-471-95512-4.
- Morimoto, Yoshiki; Kurihara, Hajime; Kinoshita, Takamasa (2000). "Can α-sultone exist as a chemical species? First experimental implication for intermediacy of α-sultone" (PDF). Chemical Communications (3): 189–190. doi:10.1039/A909094K.
- Hjelmeland, LM (November 1980). "A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis". Proceedings of the National Academy of Sciences of the United States of America. 77 (11): 6368–70. Bibcode:1980PNAS...77.6368H. doi:10.1073/pnas.77.11.6368. PMC 350285. PMID 6935651.
- "Scorecard Chemical Profile for Propane Sultone". Archived from the original on 2008-09-23. Retrieved 2008-11-17.
- "NIOSH Pocket Guide to Chemical Hazards". Retrieved 2013-11-13.