This is the current revision of this page, as edited by InternetArchiveBot (talk | contribs) at 20:53, 10 March 2024 (Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0.9.5) (Maxim Masiutin - 17955). The present address (URL) is a permanent link to this version.
Revision as of 20:53, 10 March 2024 by InternetArchiveBot (talk | contribs) (Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0.9.5) (Maxim Masiutin - 17955)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)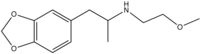
| |
| Names | |
|---|---|
| Preferred IUPAC name 1-(2H-1,3-Benzodioxol-5-yl)-N-(2-methoxyethyl)propan-2-amine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C13H19NO3 |
| Molar mass | 237.295 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
MDMEOET, or 3,4-methylenedioxy-N-methoxyethylamphetamine, is a lesser-known psychedelic drug and a substituted amphetamine. It is also the N-methoxyethyl analogue of MDA. MDMEOET was first synthesized by Alexander Shulgin. In his book PiHKAL (Phenethylamines i Have Known And Loved), the minimum dosage is listed as 180 mg. MDMEOET produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDMEOET.
Legality
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.
See also
References
- "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Archived from the original on 22 October 2017. Retrieved 12 March 2014.
External links
This psychoactive drug-related article is a stub. You can help Misplaced Pages by expanding it. |