This is the current revision of this page, as edited by Bernardirfan (talk | contribs) at 17:58, 2 May 2024 (Missing links). The present address (URL) is a permanent link to this version.
Revision as of 17:58, 2 May 2024 by Bernardirfan (talk | contribs) (Missing links)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
| IUPAC name N,N-Dinitronitramide | |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
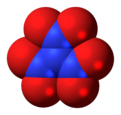
| Chemical formula | N(NO2)3 | ||
| Molar mass | 152.022 g·mol | ||
| Related compounds | |||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Trinitramide is a compound of nitrogen and oxygen with the molecular formula N(NO2)3. The compound was detected and described in 2010 by researchers at the Royal Institute of Technology (KTH) in Sweden. It is made of a nitrogen atom bonded to three nitro groups (−NO2).
Earlier, there had been speculation whether trinitramide could exist. Theoretical calculations by Montgomery and Michels in 1993 showed that the compound was likely to be stable.
Preparation
Trinitramide is prepared by the nitration reaction of either potassium dinitramide or ammonium dinitramide with nitronium tetrafluoroborate in acetonitrile at low temperatures.
- [NH4][N(NO2)2] + [NO2][BF4] → N(NO2)3 + [NH4][BF4]
Uses
Trinitramide has a potential use as one of the most efficient and least polluting of rocket propellant oxidizers, as it is chlorine-free. This is potentially an important development, because the Tsiolkovsky rocket equation implies that even small improvements in specific impulse yields a similar change in delta-v, which can make large improvements in the size of practical rocket launch payloads. The density impulse (impulse per volume) of a trinitramide based propellant could be 20 to 30 percent better than most existing formulations, however the specific impulse (impulse per mass) of formulations with liquid oxygen is higher.
References
- ^ Rahm Martin (2010). "Experimental Detection of Trinitramide, N(NO2)3". Angewandte Chemie International Edition. 50 (5): 1145–1148. doi:10.1002/anie.201007047. PMID 21268214. S2CID 32952729.
- J. A. Montgomery Jr. & H. H. Michels (July 1993). "Structure and stability of trinitramide". Journal of Physical Chemistry. 97 (26): 6774–6775. doi:10.1021/j100128a005.
- Discovery of New Molecule Could Lead to More Efficient Rocket Fuel, Science Daily, 2010-12-22, accessed 2011-01-03.
- "New molecule could propel rockets".
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |