This is the current revision of this page, as edited by Beland (talk | contribs) at 03:58, 12 May 2024 (mu not micro per MOS:NUM#Specific units and Unicode compatibility characters (via WP:JWB)). The present address (URL) is a permanent link to this version.
Revision as of 03:58, 12 May 2024 by Beland (talk | contribs) (mu not micro per MOS:NUM#Specific units and Unicode compatibility characters (via WP:JWB))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)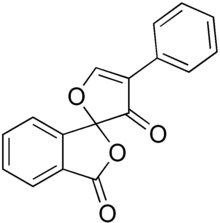
| |
| Names | |
|---|---|
| IUPAC name 4'-phenylspiro-1,3'-dione | |
| Other names Fluram | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.048.904 |
| MeSH | D005450 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H10O4 |
| Molar mass | 278.26 g/mol |
| Melting point | 153 to 157 °C (307 to 315 °F; 426 to 430 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Fluorescamine is a spiro compound that is not fluorescent itself, but reacts with primary amines to form highly fluorescent products, i.e. it is fluorogenic. It hence has been used as a reagent for the detection of amines and peptides. 1-100 μg of protein and down to 10 pg of protein can be detected. Once bound to protein the excitation wavelength is 381 nm (near ultraviolet) and the emission wavelength is 470 nm (blue). This method is found to suffer from high blanks resulting from a high rate of hydrolysis due to requiring a large excess concentration. Alternative methods are based on ortho-phthalaldehyde (OPA), Ellman's reagent (DTNB), or epicocconone.
Reaction
See also
References
- Fluram at Sigma-Aldrich
- Doetsch, Paul W.; Cassady, John M.; McLaughlin, Jerry L. (1980). "Cactus alkaloids : XL. Identification of mescaline and other β-phenethylamines in Pereskia, Pereskiopsis and Islaya by use of fluorescamine conjugates". Journal of Chromatography A. 189: 79–85. doi:10.1016/S0021-9673(00)82285-2.
- Böhlen, Peter; Stein, Stanley; Dairman, Wallace; Udenfriend, Sidney (1973). "Fluorometric assay of proteins in the nanogram range". Archives of Biochemistry and Biophysics. 155 (1): 213–220. doi:10.1016/S0003-9861(73)80023-2. PMID 4736505.
- protocol by Fluoprobes
- Biotium. "Fluorescamine PRODUCT AND SAFETY DATA SHEET" (PDF). Biotium. Retrieved 21 February 2023.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |
