This is the current revision of this page, as edited by Kku (talk | contribs) at 12:44, 14 July 2024 (link sedative). The present address (URL) is a permanent link to this version.
Revision as of 12:44, 14 July 2024 by Kku (talk | contribs) (link sedative)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Chemical compound Pharmaceutical compound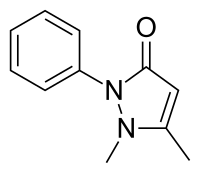 | |
| Clinical data | |
|---|---|
| Other names | analgesine, antipyrine |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 12 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.442 |
| Chemical and physical data | |
| Formula | C11H12N2O |
| Molar mass | 188.230 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Phenazone (INN and BAN; also known as phenazon, antipyrine (USAN), antipyrin, or analgesine) is an analgesic (pain reducing), antipyretic (fever reducing) and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative (as well as at least one derivative of that chemical), trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin (itself later withdrawn because of safety concerns), aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.
History
Ludwig Knorr was the first to synthesize phenazone, then called antipyrine, in the early 1880s. Sources disagree on the exact year of discovery, but Knorr patented the chemical in 1883. Phenazone has an elimination half life of about 12 hours.
Preparation
Phenazone is synthesized by condensation of phenylhydrazine and ethyl acetoacetate under basic conditions and methylation of the resulting intermediate compound 1-phenyl-3-methylpyrazolone with dimethyl sulfate or methyl iodide. It crystallizes in needles which melt at 156 °C (313 °F). Potassium permanganate oxidizes it to pyridazine tetracarboxylic acid.
Adverse effects
Possible adverse effects include:
Research
Phenazone is often used in testing the effects of other drugs or diseases on drug-metabolizing enzymes in the liver.
See also
- A/B Otic Drops, ear drops combined with benzocaine to relieve pain and remove cerumen
- Propyphenazone
References
- Jennings, Oscar (11 Jan 1890). "Antipyrin and the Prevailing Epidemic". The Lancet. 135 (3463): 105–106. doi:10.1016/S0140-6736(02)13571-9.
- Arny, H. V. (1926-09-01). "The Evolution of Synthetic Medicinal Chemicals". Industrial & Engineering Chemistry. 18 (9): 949–952. doi:10.1021/ie50201a027. Retrieved 2022-08-11.
- Schneider A, Helmstädter A (January 2015). "The evil of the unknown--risk-benefit evaluation of new synthetic drugs in the 19th century". Pharmazie. 70 (1): 60–3. PMID 25975100.
- Brune K (1997). "The early history of non-opioid analgesics". Acute Pain. 1: 33–40. doi:10.1016/S1366-0071(97)80033-2.
- Ravina E (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. ISBN 9783527326693.
- "Phenazone Concise Prescribing Info". MIMS.
- Kar A (2005). Medicinal Chemistry. New Age International. ISBN 8122415652.
- "5-Methyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one". Chemspider. Retrieved February 24, 2019.
- "Antipyrine drugs and health products". sDrugs.com.
- Chisholm, Hugh, ed. (1911). "Antipyrine" . Encyclopædia Britannica. Vol. 2 (11th ed.). Cambridge University Press. p. 134.
| Drugs used for diseases of the ear (S02) | |
|---|---|
| Infection | |
| Corticosteroids | |
| Analgesics and anesthetics | |