This is the current revision of this page, as edited by 98.191.202.231 (talk) at 02:17, 9 September 2024 (Cat.). The present address (URL) is a permanent link to this version.
Revision as of 02:17, 9 September 2024 by 98.191.202.231 (talk) (Cat.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)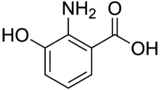
| |
| |
| Names | |
|---|---|
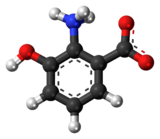
| Preferred IUPAC name 2-Amino-3-hydroxybenzoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.008.148 |
| KEGG | |
| MeSH | 3-Hydroxyanthranilic+Acid |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H7NO3 |
| Molar mass | 153.137 g·mol |
| Appearance | powder |
| Density | ≈ 1 g/cm |
| Melting point | 240–265 °C (464–509 °F; 513–538 K) decomposes 227 °C (441 °F; 500 K) from dilute HCl, decomposes |
| Solubility in water | low |
| Solubility | soluble in ether, CHCl3, alcohols |
| Solubility in hydrochloric acid | 1 N: 1 g/100 ml |
| Acidity (pKa) | at 20 °C: 1 = 2.7, 2 = 5.19, 3 = 10.12 |
| UV-vis (λmax) | 298 nm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
3-Hydroxyanthranilic acid is an intermediate in the metabolism of tryptophan. It is new antioxidant isolated from methanol extract of tempeh. It is effective in preventing autoxidation of soybean oil and powder, while antioxidant 6,7,4'-trihydroxyisoflavone is not.
References
- ^ "3-Hydroxyanthranilic acid". Santa Cruz Biotechnology, Inc. Retrieved 2015-09-24.
- ^ Armarego, Wilfred L.F.; Chai, Christina L.L. (2009). Purification of Laboratory Chemicals (6th ed.). Elsevier Inc. p. 297. ISBN 978-1-85617-567-8.
- Moline, Sheldon W.; Walker, H.C.; Schweigert, B.S. (1958). "3-Hydroxyanthranilic Acid Metabolism: VII. Mechanism of Formation of Quinolinic Acid". Journal of Biological Chemistry. 234 (4): 880–883. doi:10.1016/S0021-9258(18)70194-4. PMID 13654282. Retrieved 2015-09-24.
- Esaki, Hideo; Onozaki, Hiromichi; Kawakishi, Shunro; Osawa, Toshihiko (1996). "New Antioxidant Isolated from Tempeh". Journal of Agricultural and Food Chemistry. 44 (3): 696. doi:10.1021/jf950454t.
External links
- HAA concentration graph in lyophilized tempeh powder extract (in Indonesian)
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |