This is the current revision of this page, as edited by JWBE (talk | contribs) at 20:26, 23 September 2024 (removed Category:Phenol ethers; added Category:4-Methoxyphenyl compounds using HotCat). The present address (URL) is a permanent link to this version.
Revision as of 20:26, 23 September 2024 by JWBE (talk | contribs) (removed Category:Phenol ethers; added Category:4-Methoxyphenyl compounds using HotCat)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Not to be confused with Icaridin.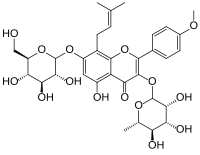
| |
| Names | |
|---|---|
| IUPAC name 7-(β-D-Glucopyranosyloxy)-5-hydroxy-4′-methoxy-8-(3-methylbut-2-en-1-yl)-3-(α-L-rhamnopyranosyloxy)flavone | |
| Systematic IUPAC name 5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-{oxy}-3-{oxy}-4H-1-benzopyran-4-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.107.649 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C33H40O15 |
| Molar mass | 676.668 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Icariin is a chemical compound classified as a prenylated flavonol glycoside, a type of flavonoid. It is the 8-prenyl derivative of kaempferol 3,7-O-diglucoside. The compound has been isolated from several species of plant belonging to the genus Epimedium which are commonly known as horny goat weed, Yin Yang Huo, and Herba epimedii. Extracts from these plants produce aphrodisiac effects, and are used in traditional Chinese medicine to enhance erectile function. However, clinical trial data are lacking to support these claims.
References
- Liu JJ, Li SP, Wang YT (2006). "Optimization for quantitative determination of four flavonoids in Epimedium by capillary zone electrophoresis coupled with diode array detection using central composite design". J Chromatogr A. 1103 (2): 344–349. doi:10.1016/j.chroma.2005.11.036. PMID 16337210.
- Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM, Shen ZY (2011). Blagosklonny MV (ed.). "Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans". PLOS ONE. 6 (12): e28835. Bibcode:2011PLoSO...628835C. doi:10.1371/journal.pone.0028835. PMC 3244416. PMID 22216122.
- Makarova MN, Pozharitskaya ON, Shikov AN, Tesakova SV, Makarov VG, Tikhonov VP (2007). "Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats". J Ethnopharmacol. 114 (3): 412–416. doi:10.1016/j.jep.2007.08.021. PMID 17890032.
- "Horny Goat Weed". Drugs.com. August 5, 2019. Retrieved November 7, 2019.
- Fang, Jian; Zhang, Yongjun (2017-10-12). "Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed". Frontiers in Pharmacology. 8: 734. doi:10.3389/fphar.2017.00734. ISSN 1663-9812. PMC 5644024. PMID 29075193.