This is the current revision of this page, as edited by M97uzivatel (talk | contribs) at 11:38, 28 October 2024 (→Synthesis: simpler link). The present address (URL) is a permanent link to this version.
Revision as of 11:38, 28 October 2024 by M97uzivatel (talk | contribs) (→Synthesis: simpler link)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Diphosphorus | |||
| Systematic IUPAC name Diphosphyne | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| Gmelin Reference | 1400241 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | P2 | ||
| Molar mass | 61.947523996 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Diphosphorus is an inorganic chemical with the chemical formula P
2. Unlike nitrogen, its lighter pnictogen neighbor which forms a stable N2 molecule with a nitrogen to nitrogen triple bond, phosphorus prefers a tetrahedral form P4 because P-P pi-bonds are high in energy. Diphosphorus is, therefore, very reactive with a bond-dissociation energy (117 kcal/mol or 490 kJ/mol) half that of dinitrogen. The bond distance has been measured at 1.8934 Å.
Synthesis
Diphosphorus has been generated by heating white phosphorus at 1100 kelvins (827 °C). Nevertheless, some advancements have been obtained in generating the diatomic molecule in homogeneous solution under normal conditions with the use of some transition metal complexes (based on, for example, tungsten and niobium). Methods for dissociation of bonds in P4 molecules via photoexcitation were also proposed.
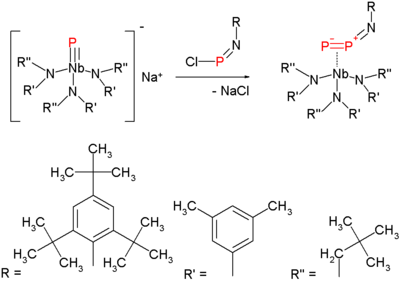
The molecule attracted attention in 2006, when a new method for its synthesis at milder temperatures emerged. This method is a variation on nitrogen expulsion in azides with formation of a nitrene. The synthesis of the diphosphorus precursor consists of reacting a terminal niobium phosphide with a chloroiminophosphane:
Heating this compound at 50 °C in 1,3-cyclohexadiene serving as a solvent and as a trapping reagent expels diphosphorus, which is reactive, as the end products are a double Diels–Alder adduct and the niobium imido compound:
The same imido compound also forms when the thermolysis is performed in toluene, but in this case the fate of the diphosphorus is unknown.
P2 has been suggested to form as an intermediate in the photolysis of P4, and in the presence of 2,3-dimethyl-1,3-butadiene the diphosphane resulting from Diels–Alder addition is again formed. To date, no direct evidence of P2 formation via P4 photolysis exists.
The generation of diphosphorus from a diphosphorus bisanthracene adduct has been reported. The synthesis of a stabilized HP2 cation has been reported.
References
- "Diphosphorus (CHEBI:33472)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- Huber, K. P.; Herzberg, G. (1979). Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules. New York: Van Nostrand. ISBN 978-0442233945.
- ^ Piro, Nicholas A.; Figueroa, Joshua S.; McKellar, Jessica T.; Cumnins, Christopher C. (1 September 2006). "Triple-Bond Reactivity of Diphosphorus Molecules". Science. 313 (5791): 1276–1279. Bibcode:2006Sci...313.1276P. doi:10.1126/science.1129630. PMID 16946068. S2CID 27740669.
- Lee-Ping Wangb; Daniel Tofana; Jiahao Chena; Troy Van Voorhisa & Christopher C. Cummins (September 2013). "A pathway to diphosphorus from the dissociation of photoexcited tetraphosphorus". RSC Advances. 3 (45). Royal Society of Chemistry: 23166. Bibcode:2013RSCAd...323166W. doi:10.1039/C3RA43940B. hdl:1721.1/90977. Archived from the original on 2017-07-21. Retrieved 2017-07-21.
- Rathenau, G. (June 1937). "Optische und photochemische versuche mit phosphor" [Optical and photochemical trials with phosphorus]. Physica (in German). 4 (6): 503–514. Bibcode:1937Phy.....4..503R. doi:10.1016/S0031-8914(37)80084-1.
- Tofan, Daniel; Cummins, Christopher C. (26 August 2010). "Photochemical incorporation of diphosphorus units into organic molecules". Angewandte Chemie International Edition. 49 (41): 7516–7518. doi:10.1002/anie.201004385. PMID 20799313.
- A Retro Diels–Alder Route to Diphosphorus Chemistry: Molecular Precursor Synthesis, Kinetics of P2 Transfer to 1,3-Dienes, and Detection of P2 by Molecular Beam Mass Spectrometry Alexandra Velian, Matthew Nava, Manuel Temprado, Yan Zhou, Robert W. Field, and Christopher C. Cummins Journal of the American Chemical Society 2014 136 (39), 13586-13589 doi:10.1021/ja507922x
- Protonation of carbene-stabilized diphosphorus: complexation of HP2+ Yuzhong Wang, Hunter P. Hickox, Yaoming Xie, Pingrong Wei, Dongtao Cui, Melody R. Walter, Henry F. Schaefer III and Gregory H. Robinson Chem. Commun., 2016, doi:10.1039/C6CC01759B
External links
- Ron Dagani, "A Mild Route To P2", Chemical & Engineering News September 4, 2006 Link
- Carmen Drahl "Flash Of Phosphorus Chemistry Innovation", Chemical & Engineering News September 13, 2010 Link
| Diatomic chemical elements | |
|---|---|
| Common | |
| Other | |