This is the current revision of this page, as edited by 2001:16b8:b418:1c00:8926:161d:c5f7:7097 (talk) at 07:05, 4 November 2024 (rv). The present address (URL) is a permanent link to this version.
Revision as of 07:05, 4 November 2024 by 2001:16b8:b418:1c00:8926:161d:c5f7:7097 (talk) (rv)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Antibiotic Pharmaceutical compound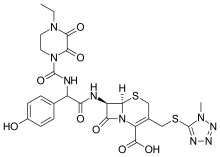 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Hepatic |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.057.936 |
| Chemical and physical data | |
| Formula | C25H27N9O8S2 |
| Molar mass | 645.67 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Cefoperazone is a third-generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics.
It was patented in 1974 and approved for medical use in 1981. Cefoperazone/sulbactam (Sulperazon) is a co-formulation with sulbactam.
Spectrum of bacterial susceptibility
Cefoperazone has a broad spectrum of activity and has been used to target bacteria responsible for causing infections of the respiratory and urinary tract, skin, and the female genital tract. The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: 0.12 - 0.25 μg/ml
- Staphylococcus aureus: 0.125 - 32 μg/ml
- Streptococcus pneumoniae: ≤0.007 - 1 μg/ml
Adverse effects
Cefoperazone contains an N-methylthiotetrazole (NMTT or 1-MTT) side chain. As the antibiotic is broken down in the body, it releases free NMTT, which can cause hypoprothrombinemia (likely due to inhibition of the enzyme vitamin K epoxide reductase) and a reaction with ethanol similar to that produced by disulfiram (Antabuse effect), due to inhibition of aldehyde dehydrogenase.
Mechanism of action
| This section is empty. You can help by adding to it. (August 2024) |
References
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 494. ISBN 9783527607495.
- "Cefoperazone (Cefobid) - The Antimicrobial Index Knowledgebase - TOKU-E". antibiotics.toku-e.com.
- Stork CM (2006). "Antibiotics, antifungals, and antivirals". In Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (eds.). Goldfrank's toxicologic emergencies. New York: McGraw-Hill. p. 847. ISBN 0-07-143763-0. Retrieved 2009-07-03.