This is the current revision of this page, as edited by Smokefoot (talk | contribs) at 16:42, 6 November 2024 (). The present address (URL) is a permanent link to this version.
Revision as of 16:42, 6 November 2024 by Smokefoot (talk | contribs) ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)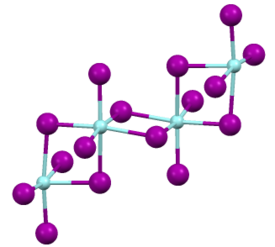 | |
| Names | |
|---|---|
| Other names zirconium tetraiodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.034.332 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ZrI4 |
| Molar mass | 598.842 g/mol |
| Appearance | orange-yellow crystalline hygroscopic |
| Density | 4.914 g/cm |
| Melting point | 499 °C (930 °F; 772 K) (triple point) |
| Boiling point | 431 °C (808 °F; 704 K) (sublimes) |
| Structure | |
| Crystal structure | Monoclinic, mP30 |
| Space group | P2/c, No. 13 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H314 |
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Other anions | Zirconium(IV) fluoride Zirconium(IV) chloride Zirconium(IV) bromide |
| Other cations | Titanium tetraiodide Hafnium tetraiodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Zirconium(IV) iodide is the chemical compound with the formula ZrI4. It is the most readily available iodide of zirconium. It is an orange-coloured solid that degrades in the presence of water. The compound was once prominent as an intermediate in the purification of zirconium metal.
Structure
Like most binary metal halides, zirconium(IV) iodide adopts a polymeric structure. As characterized by X-ray crystallography, the compound consists of octahedral Zr(IV) centers interconnected by four doubly bridging iodide ligands. The Zr-I distances of 2.692 (terminal) and 3.030 Å
Synthesis and reactions
This compound can be prepared by heating zirconium metal and an excess of iodine. The solid is purified by sublimation (400 °C, 10-4 mm Hg).
- 2 I2 + Zr → ZrI4
Pyrolysis of zirconium(IV) iodide gas by contact with a hot wire was the first industrial process for the commercial production of pure ductile metallic zirconium. This crystal bar process was developed by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925.
Heating the tetraiodide with zirconium metal gives zirconium triiodide:
- 3 ZrI4 + 4 Zr → 7 ZrI3
References
- ^ Eberly, K. C. (1963). "Zirconium(IV) Iodide". Inorganic Syntheses. Vol. 7. pp. 52–54. doi:10.1002/9780470132388.ch13. ISBN 978-0-470-13238-8..
- Krebs, B.; Henkel, G.; Dartmann, M. (1979). "Kristallstruktur von Zirkoniumtetrajodid ZrI4: ein neuer AB4-Strukturtyp". Acta Crystallogr. B35 (2): 274-278. doi:10.1107/S0567740879003344.
- Troyanov, S. I. (1986). "Crystal Structure of γ-ZrI4". Kristallografiya. 31: 446-449.
- ^ Guthrie, Dennis H.; Corbett, John D. (1981). "Synthesis and Structure of an Infinite-Chain Form of ZrI2 (α)". Journal of Solid State Chemistry. 37 (2): 256–263. doi:10.1016/0022-4596(81)90092-X.
- van Arkel, A. E.; de Boer, J. H. (1925). "Darstellung von reinem Titanium-, Zirkonium-, Hafnium- und Thoriummetall". Zeitschrift für anorganische und allgemeine Chemie (in German). 148 (1): 345–350. doi:10.1002/zaac.19251480133.
| Zirconium compounds | |||||
|---|---|---|---|---|---|
| Zr(II) | |||||
| Zr(III) | |||||
| Zr(IV) |
| ||||
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||