This is the current revision of this page, as edited by Teaktl17 (talk | contribs) at 18:54, 24 November 2024 (→top: +wl). The present address (URL) is a permanent link to this version.
Revision as of 18:54, 24 November 2024 by Teaktl17 (talk | contribs) (→top: +wl)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Chemical compound Pharmaceutical compound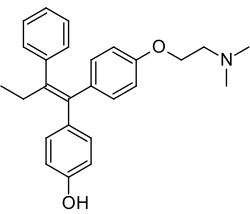 | |
| Clinical data | |
|---|---|
| Trade names | TamoGel |
| Other names | 4-Hydroxytamoxifen; 4-OHT; 4-HT; OHTAM; TamoGel |
| Routes of administration | Topical (gel) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.120 |
| Chemical and physical data | |
| Formula | C26H29NO2 |
| Molar mass | 387.523 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT) and by its tentative brand name TamoGel, is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and an active metabolite of tamoxifen. The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast. It has completed a phase II clinical trial for cyclical mastalgia, but further studies are required before afimoxifene can be approved for this indication and marketed.
Afimoxifene is a SERM and hence acts as a tissue-selective agonist–antagonist of the estrogen receptors ERα and ERβ with mixed estrogenic and antiestrogenic activity depending on the tissue. It is also an agonist of the G protein-coupled estrogen receptor (GPER) with relatively low affinity (100–1,000 nM, relative to 3–6 nM for estradiol). In addition to its estrogenic and antiestrogenic activity, afimoxifene has been found to act as an antagonist of the estrogen-related receptors (ERRs) ERRβ and ERRγ.
See also
- List of investigational sex-hormonal agents § Estrogenics
- List of selective estrogen receptor modulators
References
- ^ "Afimoxifene - BHR Pharma". AdisInsight. Springer Nature Switzerland AG.
- Desta Z, Ward BA, Soukhova NV, Flockhart DA (September 2004). "Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6". The Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1062–1075. doi:10.1124/jpet.104.065607. PMID 15159443. S2CID 21413981.
- "Statement on a nonproprietary name adopted by the USAN council: Afimoxifene" (PDF). American Medical Association. Retrieved 2008-03-26.
- ^ Goyal A, Mansel RE (16 November 2016). "Mastalgia". In Jatoi I, Rody A (eds.). Management of Breast Diseases. Springer. pp. 77–. ISBN 978-3-319-46356-8.
- Mansel R, Goyal A, Nestour EL, Masini-Etévé V, O'Connell K (December 2007). "A phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women". Breast Cancer Research and Treatment. 106 (3): 389–397. doi:10.1007/s10549-007-9507-x. PMID 17351746. S2CID 22382077.
- Prossnitz ER, Arterburn JB (July 2015). "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacological Reviews. 67 (3): 505–540. doi:10.1124/pr.114.009712. PMC 4485017. PMID 26023144.
- Levine AC (3 October 2011). Hormones and Cancer: Breast and Prostate, An Issue of Endocrinology and Metabolism Clinics of North America. Elsevier Health Sciences. pp. 271–. ISBN 978-1-4557-1239-7.
- Khetan SK (23 May 2014). "Anti-Androgenic Chemicals". Endocrine Disruptors in the Environment. Wiley. pp. 104–. ISBN 978-1-118-89115-5.
- Ariazi EA, Jordan VC (2006). "Estrogen-related receptors as emerging targets in cancer and metabolic disorders". Current Topics in Medicinal Chemistry. 6 (3): 203–215. doi:10.2174/1568026610606030203. PMID 16515477.
External links
- 4-hydroxytamoxifen at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Afimoxifene - AdisInsight
| Estrogen-related receptor modulators | |
|---|---|
| ERRαTooltip Estrogen-related receptor alpha |
|
| ERRβTooltip Estrogen-related receptor beta |
|
| ERRγTooltip Estrogen-related receptor gamma |
|
| |
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |