This is the current revision of this page, as edited by Citation bot (talk | contribs) at 01:50, 10 December 2024 (Added doi-broken-date. | Use this bot. Report bugs. | Suggested by Dominic3203 | Linked from User:Marbletan/sandbox | #UCB_webform_linked 920/2664). The present address (URL) is a permanent link to this version.
Revision as of 01:50, 10 December 2024 by Citation bot (talk | contribs) (Added doi-broken-date. | Use this bot. Report bugs. | Suggested by Dominic3203 | Linked from User:Marbletan/sandbox | #UCB_webform_linked 920/2664)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Chemical compound Pharmaceutical compound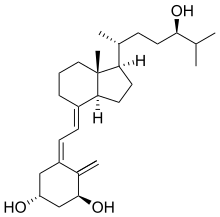 | |
| Clinical data | |
|---|---|
| Other names | (1α,24R)-1,24-Dihydroxyvitamin D3 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.855 |
| Chemical and physical data | |
| Formula | C27H44O3 |
| Molar mass | 416.646 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Tacalcitol (1,24-dihydroxyvitamin D3) is a synthetic vitamin D3 analog. Tacalcitol is marketed under several names, including Curatoderm and Bonalfa.
It is on the World Health Organization's List of Essential Medicines.
Mechanism
Tacalcitol reduces excessive cell turnover in the epidermis by interacting with vitamin D receptors on keratinocytes.
Uses
It is usually prescribed by a general practitioner or dermatologist for the treatment of psoriasis, chronic chapped lips and other severe dry skin conditions because of its ability to reduce excessive skin cell turnover. It is available as an ointment or lotion.
It has also been used for vitiligo and Hailey-Hailey disease.
References
- ^ Peters DC, Balfour JA (August 1997). "Tacalcitol". Drugs. 54 (2): 265–71, discussion 272. doi:10.2165/00003495-199754020-00005. PMID 9257082. S2CID 263503145.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- Matsumoto K, Hashimoto K, Kiyoki M, Yamamoto M, Yoshikawa K (February 1990). "Effect of 1,24R-dihydroxyvitamin D3 on the growth of human keratinocytes". The Journal of Dermatology. 17 (2): 97–103. doi:10.1111/j.1346-8138.1990.tb03714.x. PMID 2158504. S2CID 38248260.
- Fukuoka M, Sakurai K, Ohta T, Kiyoki M, Katayama I (2001). "Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes". Skin Pharmacol. Appl. Skin Physiol. 14 (4): 226–33. doi:10.1159/000056351 (inactive 10 December 2024). PMID 11464105. S2CID 24302198.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - Leone G, Pacifico A, Iacovelli P, Paro Vidolin A, Picardo M (March 2006). "Tacalcitol and narrow-band phototherapy in patients with vitiligo". Clin. Exp. Dermatol. 31 (2): 200–5. doi:10.1111/j.1365-2230.2005.02037.x. PMID 16487090. S2CID 39021489.
- Birlea SA, Costin GE, Norris DA (April 2008). "Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation". Current Drug Targets. 9 (4): 345–59. doi:10.2174/138945008783954970. PMID 18393827.
- Aoki T, Hashimoto H, Koseki S, Hozumi Y, Kondo S (November 1998). "1alpha,24-dihydroxyvitamin D3 (tacalcitol) is effective against Hailey-Hailey disease both in vivo and in vitro". Br. J. Dermatol. 139 (5): 897–901. doi:10.1046/j.1365-2133.1998.02522.x. PMID 9892963. S2CID 72418207.
| Drugs used for psoriasis (D05) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Topical |
| ||||||||
| Systemic |
| ||||||||
| |||||||||
| Vitamins (A11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fat soluble |
| ||||||||
| Water soluble |
| ||||||||
| Combinations | |||||||||
| |||||||||