This is the current revision of this page, as edited by AnomieBOT (talk | contribs) at 17:59, 17 December 2024 (Dating maintenance tags: {{Citation needed}}). The present address (URL) is a permanent link to this version.
Revision as of 17:59, 17 December 2024 by AnomieBOT (talk | contribs) (Dating maintenance tags: {{Citation needed}})(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)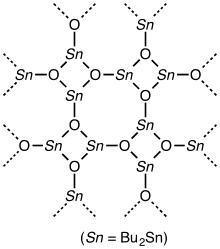
| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.011.317 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3146 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H18OSn |
| Molar mass | 248.92 |
| Appearance | white solid |
| Density | 1.6 g/cm |
| Melting point | > 300 °C (572 °F; 573 K) (decomposes 210 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms |     
|
| Signal word | Danger |
| Hazard statements | H301, H302, H315, H317, H318, H341, H360, H370, H372, H373, H410, H411 |
| Precautionary statements | P201, P202, P260, P261, P264, P270, P272, P273, P280, P281, P301+P310, P301+P312, P302+P352, P305+P351+P338, P307+P311, P308+P313, P310, P314, P321, P330, P332+P313, P333+P313, P362, P363, P391, P405, P501 |
| Autoignition temperature |
279 °C (534 °F; 552 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dibutyltin oxide, or dibutyloxotin, is an organotin compound with the chemical formula (C4H9)2SnO. It is a colorless solid that, when pure, is insoluble in organic solvents. It is used as a reagent and a catalyst.
Structure
The structure of diorganotin oxides depends on the size of the organic groups. For smaller substituents, the materials are assumed to be polymeric with five-coordinate Sn centers and 3-coordinate oxide centers. The result is a net of interconnected four-membered Sn2O2 and eight-membered Sn4O4 rings. The presence of pentacoordinate Sn centers is deduced from Sn NMR spectroscopy and Sn Mössbauer spectroscopy.
Uses
In organic synthesis, among its many applications, it is particularly useful in directing regioselective O-alkylation, acylation, and sulfonation reactions for diols and polyol. DBTO has been used in the regioselective tosylation (a specific type of sulfonation) of certain polyols to selectively tosylate primary alcohols and exocyclic alcohols over more sterically-hindered alcohols. It also finds use as a transesterification catalyst.
Dibutyltin compounds, such as dibutyltin dilaurate are widely used curing catalysts for the production of silicones and polyurethanes.
See also
References
- Davies, Alwyn G. "Organotin Chemistry", 2nd Edition, 2004, Wiley-VCH: Weinheim. ISBN 978-3-527-31023-4.
- Harris, Robin K.; Sebald, Angelika (September 1987). "The structure of polymeric dialkyltin oxides (R Me, nBu) as probed by high-resolution solid-state 119Sn NMR". Journal of Organometallic Chemistry. 331 (2): C9 – C12. doi:10.1016/0022-328X(87)80030-X.
- Beckmann, Jens; Jurkschat, Klaus; Rabe, Stephanie; Schuermann, Markus "Hexakis(2,4,6-triisopropylphenyl)cyclotristannoxane - a molecular diorganotin oxide with kinetically inert Sn-O bonds" Zeitschrift für Anorganische und Allgemeine Chemie 2001, volume 627, 2413-2419. doi:10.1002/1521-3749(200110)627:10<2413::AID-ZAAC2413>3.0.CO;2-H
- T. V. (Babu) RajanBabu, Junzo Otera "Di-n-butyltin Oxide" eEROS, 2005. doi:10.1002/047084289X.rd071.pub2
- Jorge Cervantes1, Ramón Zárraga, Carmen Salazar-Hernández "Organotin catalysts in Organosilicon Chemistry" Appl. Organometal. Chem. 2012, volume 26, 157–163. doi:10.1002/aoc.2832