This is an old revision of this page, as edited by 79.247.204.148 (talk) at 11:43, 22 December 2024 (→Preparation: A link to the recently published crystal structure of MIT has been inserted.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:43, 22 December 2024 by 79.247.204.148 (talk) (→Preparation: A link to the recently published crystal structure of MIT has been inserted.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)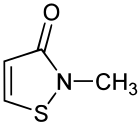
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Methyl-1,2-thiazol-3(2H)-one | |
| Other names
2-Methylisothiazol-3(2H)-one 2-Methyl-4-isothiazolin-3-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | MIT, MI |
| Beilstein Reference | 606203 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.399 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H5NOS |
| Molar mass | 115.1 g/mol |
| Appearance | white solid |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H301, H311, H314, H317, H330, H410 |
| Precautionary statements | P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P320, P321, P322, P330, P333+P313, P361, P363, P391, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Methylisothiazolinone (/ˌmɛθəlˌaɪsoʊˌθaɪ.əˈzoʊlɪnoʊn/), MIT, or MI, is the organic compound with the formula S(CH)2C(O)NCH3. It is a white solid. Isothiazolinones, a class of heterocycles, are used as biocides in numerous personal care products and other industrial applications. MIT and related compounds have attracted much attention for their allergenic properties, e.g. contact dermatitis.
Chemistry
It is prepared by cyclization of cis-N-methyl-3-thiocyanoacrylamide:
The crystal and molecular structure of methylisothiazolinone, as determined by in situ cryocrystallography, was reported for the first time in 2024.
Applications
Main article: IsothiazolinoneMethylisothiazolinone is used for controlling microbial growth in water-containing solutions. It is typically used in a formulation with 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT), in a 3:1 mixture (CMIT:MIT) sold commercially as Kathon. Kathon is supplied to manufacturers as a concentrated stock solution containing from 1.5 to 15% of CMIT/MIT. Kathon also has been used to control slime in the manufacture of paper products that contact food. In addition, this product serves as an antimicrobial agent in latex adhesives and in paper coatings that also contact food.
Hazards
MIT is allergenic and cytotoxic, and this has led to some concern over its use. A report released by the European Scientific Committee on Cosmetic Products and Non-food Products Intended for Consumers (SCCNFP) in 2003 also concluded that insufficient information was available to allow for an adequate risk assessment analysis of MIT.
Rising reports of consumer impact led to new research, including a report released in 2014 by the European Commission Scientific Committee on Consumer Safety which reported:
The dramatic rise in the rates of reported cases of contact allergy to MI, as detected by diagnostic patch tests, is unprecedented in Europe; there have been repeated warnings about the rise. The increase is primarily caused by increasing consumer exposure to MI from cosmetic products; exposures to MI in household products, paints and in the occupational setting also need to be considered. The delay in re-evaluation of the safety of MI in cosmetic products is of concern to the SCCS; it has adversely affected consumer safety.
The American Contact Dermatitis Society named methylisothiazolinone "contact allergen of the year" in 2013. The North American Contact Dermatitis Group found that methylisothiazolinone caused 10.9% positive reactions, being the third most common contact allergen in patch test results which surveyed close to 5000 contact dermatitis patients. Additionally, new research into cross reactivity of MIT-sensitized patients to variants benzisothiazolinone and octylisothiazolinone have found that reactions may occur if present in sufficient amounts.
In the United States, the Environmental Protection Agency has a 1998 data sheet on methylisothiazolinone in their Pesticides section which reads in part "Human Health Assessment: Toxicity: In studies using laboratory animals, methylisothiazolinone has been shown to be of moderate acute toxicity by the oral and inhalation routes. It is highly acutely toxic when applied dermally or to the eye and is considered to be corrosive."
Regulation
In 2014, the European Commission Scientific Committee on Consumer Safety further issued a voluntary ban on the MCI/MI mixture from leave-on products such as body creams. The measure applied for products placed on the market after 16 July 2015." Shortly thereafter, Canada moved to adopt similar measures in its Cosmetic Ingredients Hotlist.
Based on the opinion of the Scientific Committee on Consumer Safety (SCCS) of 2013, Commission Regulation (EU) 2016/1198 of 22 July 2016 amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the council on cosmetic products banned the use of methylisothiazolinone in leave-on products (skin creams and lotions) effective 12 February 2017 and limited it to 0.01% in rinse-off products (e.g. shampoo). Effective 27 January 2018 (placing on the market), the maximum concentration in rinse-off products was reduced to 0.0015%.
References
- Silva, Vânia; Silva, Cátia; Soares, Pedro; Garrido, E. Manuela; Borges, Fernanda; Garrido, Jorge (2020). "Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles". Molecules. 25 (4): 991. doi:10.3390/molecules25040991. PMC 7070760. PMID 32102175.
- Crow, W. D.; Leonard, Nelson J. (1965). "3-Isothiazolone-cis-3-Thiocyanoacrylamide Equilibria1,2". The Journal of Organic Chemistry. 30 (8): 2660–2665. doi:10.1021/jo01019a037.
- Goddard, Richard; Seidel, Rüdiger W.; Patzer, Michael; Nöthling, Nils (2024-12). "Crystal Structure of the Biocide Methylisothiazolinone". Crystals. 14 (12): 1100. doi:10.3390/cryst14121100. ISSN 2073-4352.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - Collier PJ. Ramsey A. Waigh RD. Douglas KT. Austin P. Gilbert P.; Ramsey; Waigh; Douglas; Austin; Gilbert (1990). "Chemical reactivity of some isothiazolone biocides". Journal of Applied Bacteriology. 69 (4): 578–584. doi:10.1111/j.1365-2672.1990.tb01551.x. PMID 2292521.
- "Cosmetic Ingredient Review. Final Report on the Safety Assessment of Methylisothiazolinone and Methylchloroisothiazolinone". Journal of the American College of Toxicology. 11 (1): 75–128. 1992. doi:10.3109/10915819209141993. S2CID 208506926.
- A. Schnuch, J. Geier, W. Utur, P. J. Frosch: "Patch testing with preservatives, antimicrobials and industrial biocides. Results from a multicentre study", British Journal of Dermatology, 137(3), 467–476 (1998).
- A. C. De Groot, A. Herxheimer: "Isothiazolinone Preservative: Cause Of A Continuing Epidemic Of Cosmetic Dermatitis", The Lancet, Volume 333, Issue 8633, 314–316 (1989).
- "European Scientific Committee on Cosmetic Products and Non-food Products Intended for Consumers (SCCNFP), adopted 2004" (PDF). Retrieved 31 December 2023.
- SCCS (Scientific Committee on Consumer Safety), Opinion on Methylisothiazolinone (P94) – Submission II, 12 December 2013, SCCS/1521/13, revision of 27 March 2014 (PDF)
- Castanedo-Tardana, M. P.; Zug, K. A. (2013). "Methylisothiazolinone". Dermatitis: Contact, Atopic, Occupational, Drug. 24 (1): 2–6. doi:10.1097/DER.0b013e31827edc73. PMID 23340392. S2CID 220573338.
- "Why Methylisothiazolinone-Free Cleaning Products Matter", AspenClean.com
- Schwensen, J. F.; Menné Bonefeld, C.; Zachariae, C.; Agerbeck, C.; H.Petersen, T; Geisler, C.; E.Bollmann, U; Bester, K.; D.Johansen, J (2016-06-01). "Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay". British Journal of Dermatology. 176 (1): 176–183. doi:10.1111/bjd.14825. PMID 27343839. S2CID 207075221.
- Methylisothiazolinone epa.gov October 1998
- "European Commission - Press release - Consumers: Commission improves safety of cosmetics". europa.eu. Retrieved 2016-08-10.
- "Health Canada - "December 2015 Changes to the Cosmetic Ingredient Hotlist"". 2005-09-13.
- Commission Regulation (EU) 2016/1198 of 22 July 2016 amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products, 2016-07-23, retrieved 2021-08-13
- Commission Regulation (EU) 2017/1224 of 6 July 2017 amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products, 2017-07-07, retrieved 2021-08-13
External links
- CMIT/MIT Assessment
- 2014 EPA Re-registration Review Docket with Public Commentary
- Reregistration Eligibility Decision of MIT by US EPA
- Methylisothiazoline in the Consumer Product Information Database
- Material Safety Data Sheet for product containing 0.1-1% MIT
- Commission Scientific Committee on Consumer Safety Opinion on Methylisothiazolinone (P94) Submission II (Sensitization only) SCCS/1521/13
- Methylisothiazolinone at the National Library of Medicine
- 2014 EPA Re-registration Review Docket with Public Commentary
