This is the current revision of this page, as edited by Citation bot (talk | contribs) at 03:08, 24 December 2024 (Added bibcode. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:O-methylated flavonols | #UCB_Category 17/33). The present address (URL) is a permanent link to this version.
Revision as of 03:08, 24 December 2024 by Citation bot (talk | contribs) (Added bibcode. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:O-methylated flavonols | #UCB_Category 17/33)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)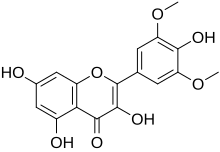
| |
| Names | |
|---|---|
| IUPAC name 3,4′,5,7-Tetrahydroxy-3′,5′-dimethoxyflavone | |
| Systematic IUPAC name 3,5,7-Trihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
3′,5′-O-Dimethylmyricetin 3′,5′-Dimethoxy-3,5,7,4′-tetrahydroxyflavone 3,5,7,4′-Tetrahydroxy-3′,5′-dimethoxyflavone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H14O8 |
| Molar mass | 346.291 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Syringetin is an O-methylated flavonol, a type of flavonoid. It is found in red grape (absent in white grape), in Lysimachia congestiflora and in Vaccinium uliginosum (bog bilberries). It is one of the phenolic compounds present in wine.
It induces human osteoblast differentiation through bone morphogenetic protein-2/extracellular signal-regulated kinase 1/2 pathway.
Metabolism
Syringetin is formed from laricitrin by the action of the enzyme laricitrin 5′-O-methyltransferase (myricetin O-methyltransferase).
Glycosides
- Syringetin-3-O-galactoside
- Syringetin-3-O-glucoside
- Syringetin 3-rhamnoside (CAS number 93126-00-2)
- Syringetin-3-O-rutinoside found in Larix sibirica
- Syringetin 3-O-(6′′-acetyl)-β-glucopyranoside found in Picea abies (Norway spruce)
References
- ^ Mattivi, Fulvio; Guzzon, Raffaele; Vrhovsek, Urska; Stefanini, Marco; Velasco, Riccardo (2006). "Metabolite profiling of grape: Flavonols and anthocyanins". Journal of Agricultural and Food Chemistry. 54 (20): 7692–7702. Bibcode:2006JAFC...54.7692M. doi:10.1021/jf061538c. PMID 17002441. S2CID 21407928.
- Guo, Jian; Yu, Dong-Lei; Xu, Lizhen; Zhu, Min; Yang, Shi-Lin (1998). "Flavonol glycosides from Lysimachia congestiflora". Phytochemistry. 48 (8): 1445–1447. Bibcode:1998PChem..48.1445G. doi:10.1016/s0031-9422(97)01025-x. S2CID 85252109.
- Lätti, Anja K.; Jaakola, Laura; Riihinen, Kaisu R.; Kainulainen, Pirjo S. (2010). "Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finland". Journal of Agricultural and Food Chemistry. 58 (1): 427–433. Bibcode:2010JAFC...58..427L. doi:10.1021/jf903033m. PMID 20000402. S2CID 28304488.
- ^ Hsu, Ya-Ling; Liang, Hsin-Lin; Hung, Chih-Hsing; Kuo, Po-Lin (2009). "Syringetin, a flavonoid derivative in grape and wine, induces human osteoblast differentiation through bone morphogenetic protein-2/extracellular signal-regulated kinase 1/2 pathway". Molecular Nutrition & Food Research. 53 (11): 1452–1461. doi:10.1002/mnfr.200800483. PMID 19784998. S2CID 42240173.
- "Laricitrin 5′-O-methyltransferase activity". AmiGO 2. Gene Ontology Consortium. 2009-02-28. Retrieved 2021-04-04.
- Foerster, Hartmut (2006-11-03). "MetaCyc pathway: Syringetin biosynthesis". MetaCyc. SRI International. Retrieved 2021-04-04.
- Matsuda, F.; Suzuki, M.; Sawada, Y. (2016-01-19). "Syringetin-3-O-galactoside; LC-ESI-QTOF; MS2; CE:Ramp 5-60 V; [M+H]+". MassBank. Retrieved 2021-04-04.
- Tohge, T. (2016-01-19). "Syringetin-3-O-glucoside; LC-ESI-QTOF; MS". MassBank. Retrieved 2021-04-04.
- ^ Slimestad, Rune; Hostettmann, Kurt (1996). "Characterisation of phenolic constituents from juvenile and mature needles of Norway spruce by means of high performance liquid chromatography–mass spectrometry". Phytochemical Analysis. 7 (1): 42–48. Bibcode:1996PChAn...7...42S. doi:10.1002/(SICI)1099-1565(199601)7:1<42::AID-PCA282>3.0.CO;2-K. S2CID 95953333.
- Tyukavkina, N. A.; Medvedeva, S. A.; Ivanova, S. Z. (1974). "New flavonol glycosides from the needles of Larix sibirica". Chemistry of Natural Compounds. 10 (2): 170–172. Bibcode:1974CNatC..10..170T. doi:10.1007/BF00563605. S2CID 4819832.
- Slimestad, Rune; Andersen, Øyvind M.; Francis, George W.; Marston, Andrew; Hostettmann, Kurt (1995). "Syringetin 3-O-(6′′-acetyl)-β-glucopyranoside and other flavonols from needles of Norway spruce, Picea abies". Phytochemistry. 40 (5): 1537–1542. doi:10.1016/0031-9422(95)00383-I. S2CID 84506810.