This is the current revision of this page, as edited by Citation bot (talk | contribs) at 11:22, 27 December 2024 (Added work. | Use this bot. Report bugs. | Suggested by Graeme Bartlett | #UCB_toolbar). The present address (URL) is a permanent link to this version.
Revision as of 11:22, 27 December 2024 by Citation bot (talk | contribs) (Added work. | Use this bot. Report bugs. | Suggested by Graeme Bartlett | #UCB_toolbar)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)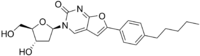
| |
| Names | |
|---|---|
| IUPAC name 3-(2-Deoxy-β-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furopyrimidin-2(3H)-one | |
| Systematic IUPAC name 3--6-(4-pentylphenyl)furopyrimidin-2(3H)-one | |
| Other names Cf1743 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H26N2O5 |
| Molar mass | 398.459 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
FV-100, also known as Cf1743, is an orally available nucleoside analogue drug with antiviral activity. It may be effective against shingles.
It was discovered in 1999 in the laboratories of Prof Chris McGuigan, Welsh School of Pharmacy and Prof. Jan Balzarini, Rega Institute, Leuven, Belgium.
Clinical trials
FV-100 was tested against valaciclovir in a phase II trial in patients with herpes zoster. The trial was sponsored by Bristol-Myers Squibb. The drug is currently being developed by ContraVir Pharmaceuticals, Inc., Edison, New Jersey. It has reached Phase III clinical trials.
References
- Inhibitex Completes Phase I Clinical Trials For FV-100 And Selects Lead HCV Compounds For Advanced Preclinical Studies, 2009
- McGuigan, Christopher; Balzarini, Jan (2009). "FV100 as a new approach for the possible treatment of varicella-zoster virus infection". Journal of Antimicrobial Chemotherapy. 64 (4): 671–673. doi:10.1093/jac/dkp294. PMID 19679595.
- Tyring SK, Lee P, Hill GT Jr, Silverfield JC, Moore AY, Matkovits T, Sullivan-Bolyai J. FV-100 versus valacyclovir for the prevention of post-herpetic neuralgia and the treatment of acute herpes zoster-associated pain: A randomized-controlled trial. J Med Virol. 2017 Jul;89(7):1255-1264. doi:10.1002/jmv.24750 PMID 27943311
- Cardiff School of Pharmacy; Pharmaceutical Sciences, Step forward for shingles drug - FV100. Shows structure of FV100.
- A Study of FV-100 Versus Valacyclovir in Patients With Herpes Zoster, 23 September 2015
- "ContraVir Pharmaceuticals: FDA Meeting About Antiviral Drug Trial.", Drug Discovery & Development, January 2015
- De Clercq E, Li G. Approved Antiviral Drugs over the Past 50 Years. Clin Microbiol Rev. 2016 Jul;29(3):695-747. doi:10.1128/CMR.00102-15 PMID 27281742
| DNA virus antivirals (primarily J05, also S01AD and D06BB) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baltimore I |
| ||||||||||||||||||||
| Hepatitis B (VII) | |||||||||||||||||||||
| Multiple/general |
| ||||||||||||||||||||
| |||||||||||||||||||||
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |