This is the current revision of this page, as edited by DMacks (talk | contribs) at 08:18, 28 December 2024 (→See also: +1). The present address (URL) is a permanent link to this version.
Revision as of 08:18, 28 December 2024 by DMacks (talk | contribs) (→See also: +1)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
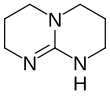
| Preferred IUPAC name 1,3,4,6,7,8-Hexahydro-2H-pyrimidopyrimidine | |||
| Other names
1,5,7-Triazabicyclodec-5-ene TBD Hexahydropyrimidopyrimidine hpp | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.024.880 | ||
| EC Number |
| ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C7H13N3 | ||
| Molar mass | 139.20 g/mol | ||
| Melting point | 125 to 130 °C (257 to 266 °F; 398 to 403 K) | ||
| Acidity (pKa) | 15.2 ± 1.0 (pKa of conjugate acid in water); 26.03 (pKa of conjugate acid in acetonitrile) | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Danger | ||
| Hazard statements | H314 | ||
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Triazabicyclodecene (1,5,7-triazabicyclodec-5-ene or TBD) is an organic compound consisting of a bicyclic guanidine. For a charge-neutral compound, it is a relatively strong base that is effective for a variety of organic transformations. TBD is colorless solid that is soluble in a variety of solvents.
Reactivity

As a strong base, TBD fully deprotonates most phenols, carboxylic acids, and some carbon acids. It catalyzes a variety of reactions including Michael reactions, Henry reactions, transesterification reactions, and Knoevenagel condensations.
Deprotonation at the 7-position gives a particularly electron-rich ligand as manifested in the redox properties of ditungsten tetra(hpp).
The conjugate acid of TBD is the preferred cation among the guanidinium hypoiodites, which are specialized oxidizing agents for various types of organic compounds.
See also
- 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN), a structurally related strong base
- 1,8-Diazabicyclo(5.4.0)undec-7-ene (DBU), a structurally related strong base
- 7-Methyl-TBD, a methyl derivative of TBD
References
- 1,5,7-Triazabicyclo[4.4.0]dec-5-ene at Sigma-Aldrich
- Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87 (4): 385–395. doi:10.5562/cca2472.
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- Huczynski, Adam; Brzezinski, Bogumil (2008). "1,5,7-Triazabicyclodec-5-ene". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rn00786. ISBN 978-0-471-93623-7.
- Pratt, Russell C.; Lohmeijer, Bas G. G.; Long, David A.; Waymouth, Robert M.; Hedrick, James L. (2006). "Triazabicyclodecene: A Simple Bifunctional Organocatalyst for Acyl Transfer and Ring-Opening Polymerization of Cyclic Esters". J. Am. Chem. Soc. 128 (14): 4556–4557. doi:10.1021/ja060662+. PMID 16594676.
- Reaction specs: initiator 4-pyrenebutanol (pyrene enables end-group determination by UV–vis) and monomer caprolactone added in ratio 1:100, targeted degree of polymerization = 100, with TBD cat. 0.5% in benzene; 72% conversion in 8 hours; polydispersity index 1.16
- Huczyński, A.; Binkowska, I.; Jarczewski, A.; Brzezinski, B. (2007). "Spectroscopic studies of the 1:1 complexes of 4-nitrophenyl(bis(ethylsulfonyl))methane and phenyl(bis(ethylsulfonyl))methane with 7-methyl-1,5,7-triazabicyclo(4.4.0)dec-5-ene and 1,5,7-triazabicyclo(4.4.0)dec-5-ene". J. Mol. Struct. 841 (1–3): 133–136. Bibcode:2007JMoSt.841..133H. doi:10.1016/j.molstruc.2007.01.005.
- Sabot, Cyrille; Kumar, Kanduluru Ananda; Meunier, Stéphane; Mioskowski, Charles (2007). "A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclodec-5-ene (TBD) under solvent-free conditions". Tetrahedron Lett. 48 (22): 3863–3866. doi:10.1016/j.tetlet.2007.03.146.
- Odagi, Miami; Nagasawa, Kazuo (2023). "Exploring Guanidinium Organocatalysts for Hypoiodite-Mediated Reactions". The Chemical Record. 23 (7): e202300030. doi:10.1002/tcr.202300030. PMID 36949010.