This is the current revision of this page, as edited by Arthurfragoso (talk | contribs) at 05:56, 2 January 2025 (Fixes image in dark mode). The present address (URL) is a permanent link to this version.
Revision as of 05:56, 2 January 2025 by Arthurfragoso (talk | contribs) (Fixes image in dark mode)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Oxalosuccinic acid" – news · newspapers · books · scholar · JSTOR (May 2019) (Learn how and when to remove this message) |
| |
| Names | |
|---|---|
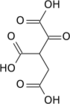
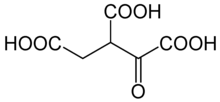
| Preferred IUPAC name 1-Oxopropane-1,2,3-tricarboxylic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.230.021 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H6O7 |
| Molar mass | 190.108 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Oxalosuccinic acid is a substrate of the citric acid cycle. It is acted upon by isocitrate dehydrogenase. Salts and esters of oxalosuccinic acid are known as oxalosuccinates.
Oxalosuccinic acid/oxalosuccinate is an unstable 6-carbon intermediate in the tricarboxylic acid cycle. It's a keto acid, formed during the oxidative decarboxylation of isocitrate to alpha-ketoglutarate, which is catalyzed by the enzyme isocitrate dehydrogenase. Isocitrate is first oxidized by coenzyme NAD+ to form oxalosuccinic acid/oxalosuccinate. Oxalosuccinic acid is both an alpha-keto and a beta-keto acid (an unstable compound) and it is the beta-ketoic property that allows the loss of carbon dioxide in the enzymatic reaction in conversion to the five-carbon molecule 2-oxoglutarate.
References
- Ochoa S (May 1948). "Biosynthesis of tricarboxylic acids by carbon dioxide fixation; the preparation and properties of oxalosuccinic acid". The Journal of Biological Chemistry. 174 (1): 115–22. doi:10.1016/S0021-9258(18)57381-6. PMID 18914069.
- Romkina AY, Kiriukhin MY (2017-04-19). "Biochemical and molecular characterization of the isocitrate dehydrogenase with dual coenzyme specificity from the obligate methylotroph Methylobacillus Flagellatus". PLOS ONE. 12 (4): e0176056. Bibcode:2017PLoSO..1276056R. doi:10.1371/journal.pone.0176056. PMC 5397045. PMID 28423051.
| Citric acid cycle metabolic pathway | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |