This is an old revision of this page, as edited by Thin Arthur (talk | contribs) at 06:54, 30 August 2007 (List of chemical compounds with unusual names). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 06:54, 30 August 2007 by Thin Arthur (talk | contribs) (List of chemical compounds with unusual names)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)|
Archives |
|
Carbenium Ions
I don't mind that you removed the mergers suggestions, but now the carbocation entry has no reference to carbenium ions. If the merger isn't going to be done, the reference to that content should still be added. I'm temporarily putting that merger back up, so if anyone was going to add carbenium ions to carbocations, then they would at least see that someone has made an entry which they can just link to instead. Ashi Starshade 06:14, 15 February 2007 (UTC)
"I don't get it: did not ever create any new content but already an authority on mergers? merge notice removed"
I have created new content, just not much lately, it's true that lately I've been suggesting mergers etc. more than new content. It seems to me that carbenium should be in the same entry as carbocation. If it's not going to be, that's fine --- but that entry still needs to be linked to it, removing the merger tag doesn't solve the real problem.Ashi Starshade 06:18, 15 February 2007 (UTC)
- Well in that case, what article within the chemistry space do you feel proud of contributing to. If you insist the merge notices will of course stay and I will post a note on the carbocation talk page V8rik 20:48, 15 February 2007 (UTC)
Proud is a strong word. I make mostly small edits because I spend my time other things, and most of them aren't even in chemistry. I am proud of being a contributor though, even though it's small. For those mergers, I agree I didn't really solve the problem either. It was just an issue of drawing attention to it so others could do it. I wouldn't want others redoing the work (e.g., writing the carbenium ion entry as a section within the carbocation entry since currently there's no connection between their entries to show the carbenium entry's existance). Ashi Starshade 03:17, 24 February 2007 (UTC)
- Thanks for your reply. I made a comment on carbocation talk page and suggest that further duscussion takes place there V8rik 22:00, 24 February 2007 (UTC)
- Thanks for the fix, I tried replying there but the server that talk page is stored on is temporarily locked. Just wanted to let you know, thanks for the fix. I appreciate it. I removed the merger tags, and you can remove this section from your discussion page now:) -Ashi Starshade 22:35, 4 March 2007 (UTC)
- Thanks and happy editing! V8rik 21:04, 5 March 2007 (UTC)
Stubs
Hi. Your comments about the use of stubs made sense to me. I'm a relatively new editor, so I'm prone to add things that aren't truly needed, like the chemistry stub I added today (I found that article via the 'Random article' nav link). Yes, it should be obvious to any reader that a particular article is short, or lacks detail. Perhaps when Misplaced Pages was new, the invitation to add content made sense, but as it matures, maybe Misplaced Pages should move away from (pleading?) with visitors and contributors to add to an article. Hurrmic 23:24, 8 January 2007 (UTC)
chemistry awards
Hi - Why did you remove the category chemistry from the Category:Chemistry awards ? --lquilter 21:55, 10 January 2007 (UTC)
- Hi lquilter, I am trying to trim down the chemistry section. I feel that to qualify for a subcategory of chemistry as one of the main sciences itself that category should at least contain 50 articles. Chemistry itself should be limited to the most important 150 articles (the Davy Medal not one of them!). Feel free to revert though V8rik 22:04, 10 January 2007 (UTC)
- Hi Varik - I'm glad you're working on the cat. The problem, though, is that in other subjects things like Awards, books, and whatnot, are categorized by their specific subject. I'm going to revert for now, but should we be talking about this with other people too? Is the chemistry project on this, or is this a developing standard somewhere? --lquilter 22:08, 10 January 2007 (UTC)
- Is this part of the effort to clean up the Category:Chemistry? I'll help with that, but I think getting rid of the subcats is the wrong way to go -- we should work on the articles. --lquilter 22:12, 10 January 2007 (UTC)
- Please revert but I do not think the categories are really a hot topic in chemistry at the moment. I am happy with the current chemistry subcats and articles so no work intended by me for now. V8rik 22:17, 10 January 2007 (UTC)
PVV
The Party for Freedom article is not improving imho, I would appreciate comments on the talk page, as I feel somewhat isolated in trying to counter what I think is pov pushing. regards --Isolani 08:57, 12 January 2007 (UTC)
Silly
Thank you for experimenting with the page Max Planck Institute for Chemical Ecology on Misplaced Pages. Your test worked, and it has been reverted or removed. Please use the sandbox for any other tests you may want to do. Take a look at the welcome page to learn more about contributing to our encyclopedia. A link to the edit I have reverted can be found here: link. If you believe this edit should not have been reverted, please contact me. Húsönd 01:49, 1 February 2007 (UTC)
- this is silly, please do not portray me as a vandal, my track record speaks for itself. The article Max_Planck_Institute_for_Chemical_Ecology does not belong in the chemistry category which should be reserved for key chemistry concepts. very recently this category was cleaned up, see discussion here: Wikipedia_talk:WikiProject_Chemistry#categorization.3F V8rik 19:15, 1 February 2007 (UTC)
Commercial suppliers
I know the links do not have a commercial intent, but also, what do they add to the article? The images are in the article, and there are non-commercial links as well. But OK, maybe worth a discussion. --Dirk Beetstra 22:49, 3 February 2007 (UTC)
- I disagree. When I include external links I do honestly believe they are worth reading. The Dean-stark link provided many technical drawings, the phosphazene links many commercially available phosphazenes and so on. I agree that in many cases commercial links should be removed especially when they simply link to the homepage and not to a relevant page but other links can be valid. V8rik 23:06, 3 February 2007 (UTC)
- I started the subject as well on the chemistry wikiproject. Indeed, the links can provide extra information, but the information is, in many cases, available from other sites as well, especially for chemicals where we now have more and more independent, searchabe databases available. In an earlier discussion the suggestion was that when there were more suppliers on the page, it would be OK, but I see mainly one or two supplier links, and indeed many links not pointing to datapages. And what does Strem tell us more about the Trost ligand, than a review on the subject (the article is unreferenced ...)? But I'll wait for some more input on this matter. --Dirk Beetstra 23:13, 3 February 2007 (UTC)
Supramolecular analytical chemistry
I like the content concerning supramolecular analytical chemistry that you added to the supramolecular chemistry page. However, might I suggest that we start a new page for the content. Here is my reasoning. First I think that it is a large area of research that rightly deserves its own page. Second I think that the supramolecular chemistry page should remain relatively broad and direct people to more specific areas.
I would suggest that the new article was entitled molecular sensors as I believe this is the more common name for this area and such a article is obviously lacking. It appears that you put some time into the new content and I wanted to consult with you first before making revisions. M stone 22:37, 8 February 2007 (UTC)
- Hi M stone, I am not sure. I do not intent to expand the segment any further so it will remain small, also the supramolecular chemistry article did lack any to the point applications that is why I put the segment there. But if you think it should move then move it V8rik 19:37, 9 February 2007 (UTC)
- I think that I can expand the content that you have added about analytical supermolecular chemistry to make a good article. I think that the supramolecular chemistry should be enriched however I am not in favor of adding specific examples for such a broad area. Instead I would like to see it closer to the chemistry page where there are short descriptions of important topics within the area and that link to the appropriate pages. M stone 00:04, 10 February 2007 (UTC)
- the content has now vanished? Be adviced that the review article I based my articlet on never mentions molecular sensors, and I dont want the NOR police on our tail. By the way great work on your recent images, the supramolecular section really needed those. V8rik 13:47, 10 February 2007 (UTC)
- I moved the content to Molecular Sensor unfortunately I accidentally capitalized "Molecular" so it won't link unless the link is capitalized as well. Not sure how to fix but I will figure it out. Your point is well taken that they never actually use the term molecular sensor. It only describes sensors that are molecules. Probably best to add a few more references. Thanks about the pictures. M stone 16:13, 10 February 2007 (UTC)
- solved that problem for you: i simply moved the article to Molecular sensor, thanks for the collaboration V8rik 22:13, 10 February 2007 (UTC)
Chemistry
V8rik, can you provide the name of the molecule image you have on the chemistry page. Thanks: --Sadi Carnot 16:58, 13 February 2007 (UTC)
- Hi Sadi Carnot, I did not put that image there but the only name I have for you is Taxol see: Taxol total synthesis you can find the very long systematic name on the Taxol page. V8rik 21:22, 13 February 2007 (UTC)
- Thanks: --Sadi Carnot 22:51, 8 March 2007 (UTC)
Missing topics in chemistry
Thanks for help with the missing topics page - Skysmith 10:07, 16 February 2007 (UTC)
Macromolecule
I'm not sure I understand your comment. I removed the IUPAC recommendation stuff because it is largely irrelevant to the existing article, redundant with and/or contradictory with existing article text, and factually incorrect. The IUPAC page that you link to describes the use of the term macromolecule in the context of polymer science only to disambiguate between single polymer molecules (a macromolecule) and substances comprised of macromolecules (a polymer). I have removed your content and posted a discussion on the talk page. In any event, the wording chosen for the wikipedia entry is too close to the copyrighted material in the IUPAC recommendation and may be considered plagiarism (unless it is included in quotes). Please respond to this and allow us to achieve consensus before reinserting the content. Irene Ringworm 18:45, 23 February 2007 (UTC)
- Please do not delete referenced material. It is harmful. See also my user page for a general comment on deletions. V8rik 18:56, 23 February 2007 (UTC)
- It is copied verbatim from the IUPAC website and may constitute copyright infringement. It is also referenced without full context and is factually incorrect as written. Please follow the discussion on the talk page and do not reinsert the copyrighted content until consensus has been achieved on the talk page.
In the meantime I have commented out your addition (not deleted it) so that it can be quickly reinserted if consensus warrants. In the meantime, copyright concerns trump a wish to not delete referenced material. Irene Ringworm 19:16, 23 February 2007 (UTC)
- Not interested in a long revert war. Please rework your content so that it does not violate copyright or it will be removed. Irene Ringworm 19:26, 23 February 2007 (UTC)
- your choice: an active and productive Wiki editor or nothingness. I am already prohibited from editing political articles due to the poor quality of editors in that section. V8rik 19:28, 23 February 2007 (UTC)
- I appreciate your willingness to contribute but you can't just copy and paste stuff you find on the IUPAC website without violating their copyright. Please follow the talk page discussion to achieve consensus or submit non-copyrighted, factually accurate material. Irene Ringworm 19:31, 23 February 2007 (UTC)
- Note also that your last hasty revert undid my added content which attempted to work the IUPAC definition into the main definition. Please follow the talk page discussion. Irene Ringworm 19:33, 23 February 2007 (UTC)
- I have rewritten the IUPAC material in my own words to clear up both the factual errors and copyright issues posed by your revert. Please resort to normal dispute recognition mechanisms such as the talk page (to which you have not responded) rather than meaningless edit wars to reinsert content that clearly violates copyright. Please review the WP:3RR policy carefully before considering future reverts. In the meantime, I think that I would prefer nothingness. Irene Ringworm 20:09, 23 February 2007 (UTC)
Opened merge discussion on Isotactic
Though that there would be no controversy for merge on isotactic. Feel free to weigh in on the talk page. Irene Ringworm 19:37, 25 February 2007 (UTC)
- Aha! Read your user page on the whole merge philosophy. I still think that this one is a no-brainer, even by your criteria. Irene Ringworm 19:42, 25 February 2007 (UTC)
- Thanks for taking time to read my 2 cents on mergers in general. I will make a comment on your merge proposal on the releavant page. V8rik 21:10, 25 February 2007 (UTC)
- So I want to understand the depth of your convictions. I have also proposed a merge on terpolymer, a page which has absolutely no possibility for expansion whatsoever. What are your thoughts here? Irene Ringworm 18:18, 26 February 2007 (UTC)
- I guess there is no stopping you from merging the whole of polymer chemistry to one big ugly 100kb+ article. We will end up merging humans with the solar system because after all humans are unique to earth and earth is unique to etc. Just take a look at the polymer chemistry category: in my opinion it should contain lets say 50 key topics in polymer chemistry and topics people regularly want to look up (same for polymer physics). If you have your way the category will cease to exist but if I have to select an encyclopedia the first thing I do is look at the index and if am not impressed I will look for another. I think the focus should be on adding content to Wiki, sure terpolymer is a minute topic but graft copolymers for which you also propose a merge is huge. I do not oppose your mergers any further but do not expect me to contribute any further to polymer science either, good luck with it. V8rik 22:41, 26 February 2007 (UTC)
- Come on, now. I'm approaching you in good faith. For example, I agree with your concern about degree of polymerization being buried in some humongous article on polymerization. I've also been working in other areas to unmerge crazy redirects (see x-ray diffraction, which was for some reason merged with x-ray crystallography). I would also never consider merging tacticity into polymer or stereochemistry. I do think, however, that we can merge isotactic, atactic, and syndiotactic into tacticity with very few problems. In this case it's easy to solve your "buried in some gargantuan article" problem by (a) defining and bolding isotactic, atactic, and syndiotactic in the first few sentences of the tacticity heading and (b) Creating well-defined sub-sections accessible from the heading. Same goes for terpolymer, which could easily be bolded and defined in the introductory paragraph of copolymer. This doesn't work for degree of polymerization because it doesn't make sense to have this term in the first few paragraphs - it would certainly be buried in a longer article about synthetic polymer chemistry. As for graft copolymer, my merge proposal is less based on cleanup and more based on the fact that the article is an unencyclopedic, fact-free piece of garbage (see the talk page). Feel free to respond - that's why I've opened discussion on the talk page.
- What I'm really trying to figure out is how strongly you feel about this. You seem opposed to merges on a matter of principle. Will any merge proposal be met with an immediate opposition or are you open to discussion? I'm not interested in spending the time to clean up the area and build consensus if you simply plan to revert any merger based on the notion that merge=bad.
- As for adding content, if you have any responses to my improvement drive for the main polymer article your input is welcome. Irene Ringworm 23:10, 26 February 2007 (UTC)
- Well, I am not against mergers perse, recently I proposed a merge for atomos to atom (this IS a dictionary item) and Theoretical yield to yield (chemistry). I also redirected countless links. I will revert deletions vehemently because I think Wiki should be preserved but I usually respect mergers after due discussion. I only choose not to invest more time in polymer chemistry because of the rate at which mergers are being proposed and supported and that is not the direction I would like to see it going. I want to enjoy this work and I noted i was getting upset. So lets say I take a break from polymers and happy editing V8rik 22:26, 27 February 2007 (UTC)
- Just don't take a break for too long. I haven't published anything in polymer science for nearly a decade and I'm a little bit rusty. Having someone looking over my shoulder will keep me honest. Irene Ringworm 00:26, 28 February 2007 (UTC)
PSEPT Wades Rules article
Hi there!
This article sprang from electron counting and the formulae have been unchanged from Day 1. I am contacting you in the hope that you will be able to confirm my suspicions and point me at the right authority (assuming there is one!)on boranes/clusters. There seems to be a typo in the rules-- for example I cannot make them work with simple nido and arachno boranes. e.g nido B5H9 and arachno B4H10. I think the nido formula should be 2n + 2(n+2) giving 24 the correct valence electron count for B5H9; and the formula for arachno should be 2n + 2(n+3) giving for e.g B4H10 22 electrons. These formulae seem to work with carboranes OK. I have my doubts about the transition metal formulae too. Axiosaurus 15:24, 28 February 2007 (UTC)
Hi Axiosaurus, you are right, thus far the article Polyhedral skeletal electron pair theory escaped a critical review and proper citation. I vaguely remember that some content did not match with Jolly but I left it at that. I will move the article to the top of my to-do list maybe I can sort some of it out. I am not the expert you are looking for! I find it one of the most challenging topics in chemistry to tackle and for my it is always a struggle to make sense of it V8rik 22:31, 28 February 2007 (UTC)
I have got too much time on my hands at the moment in forced reirement, so I shall bravely go where perhaps I shouldn't and edit this one, on the basis that an aunt sally will provoke (constructive?) criticism. When its done I will get in touch with my old theoretical chemistry lecturer, Brian Duke, who I see is an active editor. I know that he worked with boranes at one stage long long ago. Axiosaurus 12:18, 1 March 2007 (UTC)
- Welcome to the team then! I am looking forward to your edits V8rik 17:27, 2 March 2007 (UTC)
hi again, I've rewritten the article. see what you think. Axiosaurus 22:00, 3 March 2007 (UTC)
- Looks great!. I have made one edit with respect to introducing the concept (the title of the article must always return in bold in the first sentence, every article in wiki does that). I also linked to cluster chemistry. The only thing lost in the new edit compared to the previous edit is what the different types of clusters (nido, closo arachno) are really like. Do you have suggestions? V8rik 22:57, 3 March 2007 (UTC)
hi, Good. I left out the closo nido because I didn't like the words too much. However surprisingly the terms are not defined anywhere else! So you're right! I need to put something in. Looking at the borane categrory and boron compounds the level of documentation is sparse. My current view is that the article Borane should be expanded to become a family page with a proper overview of all BxHy compounds..with all the right words--e.g. deltahedra, closo etc. etc. I'll contact others on the Borane talk page to see what the concensus is. Axiosaurus 14:50, 4 March 2007 (UTC)
History of Benzene
I tried to edit "benzene" and you reversed my edits, because they were mostly deletions. Perhaps I don't understand Wikepedia yet, and in fact this was my very first attempt at editing an article. I am a professional historian of chemistry whose expertise is exactly this, namely the history of organic chemistry, especially in 19th century Germany. I have widely published on this material, over thirty years. You say that my deletion was damaging. I say that the information that I deleted was misleading and/or irrelevant, in other words, damaging. How can I convince you of that? Ajrocke 21:18, 7 March 2007 (UTC)
- Hi Ajrocke, thanks for your comment. With your background you can make a great contribution to chemistry so welcome! let me explain my quick reversal:
- for information on how to cite and formats: Misplaced Pages:Scientific citation guidelines. One thing it asks of editors to specify what content in an article is controversial. Other editors can then try to find references
- be patient: collecting the references takes time and again my point try to focus on the controversial material first
- the material on Archibald Scott Couper is not referenced on the page itself but on its bio page, its 1911 but it still counts
- why throw out the snake stuff. if you find it controversial tag it with and other editors may find a reference for you. Even if it is untrue I am still interested why these untruths persist.
- if misleading: rewrite but not delete
- relevance is difficult to judge in Wiki. See NPOV guidelines. What is irrelevant to you perhaps is relevant to me. deletion is not an option
To me Wiki editing is about creating content and fact checking. Deleting material is the opposite. If you try to motivate your deletions I see no reason for any further reverts. I am looking forward to your edits.
V8rik 21:53, 7 March 2007 (UTC)
Hi, V8rik. I understand. I've made another effort, please check it out.
Ajrocke 15:07, 8 March 2007 (UTC)
- It looks great! Yesterday we discussed deletions but you did a great job expanding the article. I can imaging now that you considered the article misleading with respect to the thirsty Germans but you have set the record strait. Have you considered adding citations? The kekule dream is covered in my Morrison&boyd but briefly and my next trip to the library will be a while. JCE only yielded history of aromaticity and history on cyclohexane. No luck there V8rik 22:03, 8 March 2007 (UTC)
- Hi, V8rik. Thanks. At your suggestion, I've now added a bunch of references. If you're interested in the dream story about benzene, take a look at how I've added substantially to the Kekule article. I used some language from my benzene edits for my expansion of the Kekule article -- I assume that's OK in Wiki-land. It's my language, and it's original to me (but it's not original research! -- it's all from published sources in well-refereed journals).
Ajrocke 19:00, 9 March 2007 (UTC)
- Thanks for the references. I also noted todays edit. Please note that articles exist on benzoin and benzoin resin which are not really related. To what benzoin do you refer? V8rik 22:07, 10 March 2007 (UTC)
- I mean benzoin resin. I'll change it right now to disambiguate. The new edits by Stone are good additions. Ajrocke 19:11, 12 March 2007 (UTC)
- Thanks, that sorted out then V8rik 21:27, 12 March 2007 (UTC)
Tagged images
V8rik just to let you know that I've tagged a few of your chemical images with {chemical drawing required} - as was suggest on the on-going message on the chemical project page about coloured-images. Overall, like you, I think basic images are fine - but if someone is keen to re-draw these images at higher resolution then I say let them! -- all the best Quantockgoblin 10:03, 3 April 2007 (UTC)
- fine with me! I may even update them myself at a convenient time V8rik 20:29, 3 April 2007 (UTC)
Carboxyl group
Hi! I re-stubbed it again, what do you think? should it still be a redirect to Carboxylic acid?--The Joke 15:03, 11 April 2007 (UTC)
Image:Fulvalene.png listed for deletion
An image or media file that you uploaded or altered, Image:Fulvalene.png, has been listed at Misplaced Pages:Images and media for deletion. Please look there to see why this is (you may have to search for the title of the image to find its entry), if you are interested in it not being deleted. Thank you. —Bkell (talk) 07:00, 24 April 2007 (UTC)
- if somebody else chooses to replace this image with somthing else thats alright with me but that person shuld also deal with the orphaned image. V8rik 17:30, 24 April 2007 (UTC)
In response to your comments on aldol condensation
I am not aware of any rules or policies that say an article has no responsibility to provide context or explanation. I am however, aware of things such as WP:PERFECT which clearly state
* is understandable; it is clearly expressed for both experts and non-experts in appropriate detail, and thoroughly explores and explains the subject. * is nearly self-contained; it includes essential information and terminology, and is comprehensible by itself, without requiring significant reading of other articles.
There is also the featured article criteria to consider, which says
* "Comprehensive" means that the article does not neglect major facts and details.
And Misplaced Pages:Article development
Start your article with a concise lead section or introduction defining the topic at hand and mentioning the most important points. The reader should be able to get a good overview by only reading the lead, which should be between one and four paragraphs long, depending on the length of the article. See Misplaced Pages:Lead section.
Remember that, although you will be familiar with the subject you are writing about, readers of Misplaced Pages may not be, so it is important to establish the context of your article's subject early on.
among other things. Instead of commenting on me (WP:NPA), why don't you try improving this article? It would be much more effective. Thus I will re-add the clean-up templates since the concerns I expressed are unfixed. Mister.Manticore 20:48, 25 April 2007 (UTC)
- I am retracting my support to both articles, have them removed if you like. Misplaced Pages does not offer any defence to your practices which is unfortunate V8rik 21:08, 25 April 2007 (UTC)
- What removal? I'm suggesting their improvement, nothing more. That's not a bad thing, and shouldn't be treated as such. Mister.Manticore 21:24, 25 April 2007 (UTC)
Chenodeoxycholic acid based molecular tweezers
Would you be opposed to the removal of the example of molecular tweezers based on a chenodeoxycholic acid scaffold? My concern is that it is from the Bulletin of the Korean Society and that it is not a particularly important example. For instance, I have not found it included in any reviews of molecule tweezers. I wanted to check with you before I removed it. I know that in general you are opposed to deleting content, but I think that in this case it may be justified. M stone 11:31, 28 April 2007 (UTC)
- Hi M stone, yes I am generally opposed to deletions. I also think that when a concept requires an example any example will do regardless the quality of the journal. If you advocate the rule IF NOT IN REVIEW = DEL I would be disappointed as well. On the other hand it detonates with your beautiful buckycatcher image (my thanks!) and I am in a good mood so therefore I have moved the content myself. V8rik 17:32, 30 April 2007 (UTC)
- I just wanted to discuss the change before doing anything. I have the upmost respect for editors that actually contribute content. I definitely don't believe in hard rules since they impede creativity! M stone 21:40, 30 April 2007 (UTC)
- So we agree on the main point about creativity great! Thanks V8rik 21:03, 1 May 2007 (UTC)
Isotactic Polymer image
Any chance we can replace it with This copy? ♥♥ ΜÏΠЄSΓRΘΠ€ ♥♥ 20:15, 2 May 2007 (UTC)
- by all means go ahead, much better image quality V8rik 21:19, 3 May 2007 (UTC)
Missing double bond?
Just wanted to let you know that  seems to be one double bond short! • TheBendster (talk) 7 May 2007, 08:05 (UTC)
seems to be one double bond short! • TheBendster (talk) 7 May 2007, 08:05 (UTC)
- Hi TheBendster, thanks for spotting the error, I have made a correction. V8rik 19:56, 7 May 2007 (UTC)
Chemical equilibrium
I should appreciate your comments on the modified proposal at talk:chemical equilibrium#new draft articlePetergans 11:17, 7 May 2007 (UTC)
- Hi Petergans, thanks for the invite but I find it difficult to make sense of it, better take it one step at the time. Simply replacing one article altogether is highly unusual. I have made my comments on the talk page V8rik 19:49, 7 May 2007 (UTC)
My main concern has been to get the science right. I did not undertake to replace the article lightly. Here is a summary of my reasons for doing so.
- Chemical equilibrium and equilibrium constant are core articles referred to by many other articles. When I first looked at these articles I thought about incremental edits but soon realised that they contained too many fundamental misconceptions for this to be possible. Regarding the old chemical equilibrium article:
- The treatment of activity is fundamentally unsound. What nobody seems to realise (and I include many text-book writers in this) is that in the modern era solution equilibrium constants have usually been measured using solutions at high ionic strength. In that case the concentration quotient is not an approximation, but is a thermodynamic quantity in its own right. This is discussed in full in Rossotti and Rossotti (1961); not exactly recent stuff!
- Explanations in terms of kinetics are wrong. The “force” driving a mixture to equilibrium is the tendency to minimise the free energy. It is true that entropy will tend to a maximum in any spontaneous process, but the value of the equilibrium constant depends also on the enthalpy of the reaction, so the equilibrium concentrations will depend on both enthalpy and entropy.
- The equality of forward and backward reaction velocities at equilibrium is a consequence of ΔG=0, not the other way round. The original argument of Guldberg and Waage is correct only for certain simple reactions as the rate laws of chemical kinetics do not, in general, follow the stoichiometry of the reaction. Equilibrium constants, on the other hand, always do so. Kinetics are essentially irrelevant to equilibria since it matters not a whit how quickly of slowly equilibrium is reached.
- The section on thermodynamics is not particularly relevant. In Thermodynamic equilibrium the necessary conditions for equilibrium are given correctly. It is not necessary to minimise the free energy change by a minimisation technique since the value of ΔG at equilibrium is zero.
- There is no derivation of either the activity quotient or the concentration quotient. ΔG=- RT ln K is stated with any justification.
- Belousov-Zhabotinsky reaction. This is not an example of equilibrium; it’s blatantly a kinetic thing (oscillating reaction). This illustrates how fundamental misconceptions creep into these articles.
- Given these issues, I concluded that each paragraph would need to be re-written, that is, the article needs to be replaced. I know a lot of people have contributed in the past but it seems to me that none of them were expert. On the other hand, I have more than 30 years’ research and teaching experience in the field of solution equilibria, but am a newbie in regard to writing for Misplaced Pages.
- I wanted to expand the article considerably, but could not see a way to do that in the framework of the old article.
You said before that you did not follow the thermodynamics. My reply has to be that thermodynamics is fundamental to understanding equilibria. This was already implied when Guldberg and Waage used the term "active mass", but could not explain what the active bit meant. One could give a qualitative explanation of equilibria without thermodynamics, but since equilibrium constants are quantitative that would not be enough. By the way, I took your comment about SO3 on board by creating the metastable subsection.
Petergans 08:43, 17 May 2007 (UTC)
- Thank for your comment, but I prefer the discussion concentrated in chemical equilibrium talk. I will wait and see what other comments accumulate before taking actions. V8rik 21:34, 17 May 2007 (UTC)
Image:150px-Borneol.png listed for deletion
An image or media file that you uploaded or altered, Image:150px-Borneol.png, has been listed at Misplaced Pages:Images and media for deletion. Please look there to see why this is (you may have to search for the title of the image to find its entry), if you are interested in it not being deleted. Thank you. Isilanes 17:32, 13 May 2007 (UTC)
Aryllead reaction
The diagram you've added for the lead arylation of a beta keto ester has the carbonyl in the wrong spot. I'd edit it myself but I'm not sure how, or whether it is good Misplaced Pages etiquette or whatnot... but just so you know, in the depicted lead arylation the ketone and ester should be beta to one another, not gamma.
Laurbrown 09:13, 22 May 2007 (UTC)
- Thanks Laurbrown, for pointing out the mistake, I do not know what the Wiki rule book says but as far as I am concerned I made the mess and it was up to me to clean it up. Thanks V8rik 19:42, 22 May 2007 (UTC)
Catalysis
I don't think that what you have written about enzyme catalysis is correct. As I understand it an enzyme catalyses the forward reaction by lowering the potential barrier for that reaction. Take the case of carbonic anhydrase. It speeds up the reaction CO2+H2O HCO3 + H by bringing the reactants into close proximity. The backward reaction is fast anyway. So, the enzyme speeds up only the forward reaction so that equilibrium is reached quickly. The backward reaction has a quite different reaction profile. For one thing, the ions carry opposite charges so they will be easiliy attracted to each other, whereas CO2 is hydrophobic.
If the sources you cite say different, they are, in my opinion, wrong. Another text-book error?
BTW, thanks for starting to clean the article up. Petergans 20:14, 25 May 2007 (UTC)
Think about this some more, I wonder if a catalyst only ever acts on one reaction. In the case of N2+3H2 2NH3, the activation energy is very high because of the very large amount of energy required to break an N-N bond. In the reverse reaction much less energy is required to break an N-H bond, so the activation energy is much lower. A catalyst works by reacting with the reactants (substrate for an enzyme) to form an intermediate compound which then goes on to form the products rapidly. The activation energy for the reaction forming the intermediate is much lower than it is for the direct reaction. A good example is the old lead chamber process where the oxides of nitrogen react with SO2; in that case the intermediate compound has been identified and characterised. I don't know for sure that we can make a general statement, but it looks that way to me now. Petergans 08:44, 26 May 2007 (UTC)
- Agreed, my references did not specifically mention enzymes only catalysts. I modified the text accordingly. I know you are trying to convince me that the textbooks I have cited are wrong about the catalyst effect but I am still puzzled. What about microscopic reversibility?. You make it sound as if you can design a Haber catalyst that will make the reaction go to completion. If you can point me to literature proving your case I am happy to go to the library and read up on it V8rik 17:31, 26 May 2007 (UTC)
My point is only that I believe that a catalyst speeds up either forward or backward reaction, but not, in general, both. Fundamentally I think that a catalyst works for a specific reaction by providing an alternative mechanism, which has a lower activation energy, by which that reaction can proceed . I imagine that the reaction 2NH3 N2+3H2 is quite fast in the absence of a catalyst at the temperature used in the Haber process, but I don't have any evidence to back up this idea.
I see that this appears to conflict with the principle of microscopic reversibility. Well, I don't think that it does because in a multi-step reaction mechanism the rate-determining step is different for forward and backward reactions.
I can't at the moment give you a literature reference because I can't remember ever reading about this. All discussions of catalysis that I've read talk in terms of a single reaction, not an equilibrium system. So, I've posted a question at the reference desk/science to see if anyone can come up with a citable source that we can use. Petergans 18:59, 26 May 2007 (UTC)
- Okay, we will give the topic a rest for now and wait for developments V8rik 20:31, 26 May 2007 (UTC)
Have a look at haber process#catalysts. The rate-determining step in the forward reaction is the dissociation of N2 on the catalyst surface. I imagine that this is irrelevant for the backward reactions in which ammonia dissociates starting with . N2 can easily be generated in the gas phase once there are radicals flying around. Certainly the reaction does not need a catalyst. Is there a detailed kinetic study of ammonia dissociation in the literature?
There are two replies to my query at Misplaced Pages:Reference_desk/Science#catalysis and equilibrium which have clarified things a little: in some reactions the catalyst does affect both forward and backward reactions. Nevertheless, I am not convinced that it always true. Petergans 08:39, 27 May 2007 (UTC)
Afd Notice - second time around for Unsolved problems in chemistry
Unfortunately this article has again be nominated for deletion Misplaced Pages:Articles for deletion/Unsolved problems in chemistry. I know you tried to save it the first time around. Could you please help again. Heliumballoon 17:31, 30 May 2007 (UTC) Hi Heliumballoon, I have made my case in Wikipedia_talk:WikiProject_Chemistry but I will not participate in the nomination procedure. Some democratic rights are violated. V8rik 17:35, 30 May 2007 (UTC)
Thermodynamics
What is your problem with the thermodynamics? I followed the standard derivation for ΔG=-RT ln K as given in Atkins' Physical Chemistry Editions 1 and 8, where K is the reaction quotient at equilibrium. Put simply, the chemical potential of reactants is set equal to the chemical potentials of the products. Atkins defins chemical potential as
chemical potential gives a virtually identical expression. This shows that chemical potential is a partial derivative of the Gibbs energy. It can be used to find the minimum where the slope of the Gibbs energy with respect to the reaction coordinate is zero, that is, the derivative of G is zero. I took the view that the definition of chemical potential should not be repeated in chemical equilibrium, but it is debatable that the presentation is too concise. Your recent additions to the text regarding thermodynamics, however, are without merit and should be removed. Petergans 09:54, 31 May 2007 (UTC)
- Hi Petergans, thanks for your comment, I think this discussion belongs on the chemical equilibrium Talk:Chemical_equilibrium so I will leave ny comment there. V8rik 17:44, 31 May 2007 (UTC)
Images listed for deletion
Some of your images or media files have been listed for deletion. Please see Misplaced Pages:Images and media for deletion if you are interested in preserving them.
Thank you. isilanes (talk|contribs) 20:27, 31 May 2007 (UTC)
Organocatalysis & related
I just thought i'd drop by to suggest that perhaps we just get the re-vamp of the article under way, as Mike seems more of the defensive type. I've explained my field was NMRS and I'll leave any further expansion to him. Just as a note; is english his native language? ♥♥ ΜÏΠЄSΓRΘΠ€ ♥♥ 20:09, 2 June 2007 (UTC)
- Hi Minestrone Soup, I made my statement on organocatalyst talk and I have little to add. I am rarely seeing new content and tend to give new people benefit of the doubt as long as new material is coming in and in fact I am confident that eventually a proper article will emerge. With respect to language, I do not have any concerns. I myself am not a native English speaker but I am happy with my googlebar spell checker. In my experience there are plenty people volunteering to translate globish to English V8rik 20:51, 2 June 2007 (UTC)
Just a suggestion for further compound images
If you're going to make any further compound or structural images, would you perhaps try using BKChem ? It's open-source and can export images to SVG format, and saves a lot of hassle drawing them out. ♥♥ ΜÏΠЄSΓRΘΠ€ ♥♥ 20:47, 3 June 2007 (UTC)
- Let me suggest ChemTool. Free software and exports to SVG. — isilanes (talk|contribs) 20:56, 3 June 2007 (UTC)
- Also good, but i chose BKChem because it's usable on all Operating systems :-) ♥♥ ΜÏΠЄSΓRΘΠ€ ♥♥ 20:57, 3 June 2007 (UTC)
- Thanks Minestrone Soup, I decided to give it a go and I am very happy with the graphic. the editing in BKChem requires more maneuvering but it requires getting used to I guess. I will continue to explore its options!
on the other hand it seems the applic is unable to handle subscripts in svg:
the image before that was just a lucky one, the jpg export still works
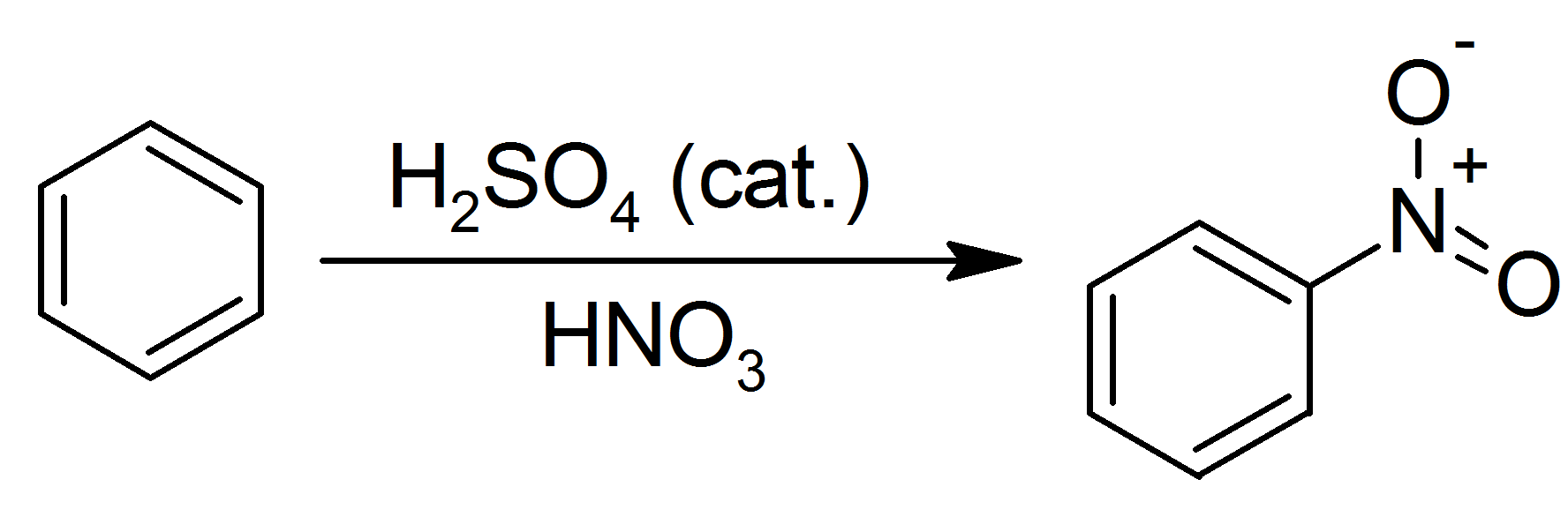
- Nope, that's Misplaced Pages's SVG->PNG thumb generator. For example, for your Benzene Nitration diagram, take a look at This URL :-) ♥♥ ΜÏΠЄSΓRΘΠ€ ♥♥ 23:16, 21 July 2007 (UTC)
Metastable mixtures
Just to let you know that I intend to revise this section, replace the SO2/SO3 example by Haber-Bosch ammonia, and possibly rename the section "Effect of catalysts". I'm away at a conference for the rest of this week. Petergans 20:55, 4 June 2007 (UTC)
- I expressed my doubts on this section before: I have references that include the sulfur dioxide - oxygen equilibrium and the Haber equilibrium as regular equilibria, there is really nothing out of the ordinary to observe. See for example Atkins/Jones chemical principles A quest for insight. It is not totally explicit in their text but it is suggested that the SO2 equilibrium is the one studied by Waage/Cato in the first place. The one reference I have found explains metastability with respect to equilibria in this way:
metastable state: if variables of state are changed very rapidly in comparison to time required for the establishment of equilibrium the system cannot follow these changes. The original state is frozen in at a different temperature of pressure. Examples: crystals, glasses and supersaturated vapors. (from the concise encyclopedia chemistry). No practical example is given though.
It seems your definition of a metastable state is one in which a chemical reaction is just very slow requiring to overcome a kinetic barrier but the general view is that any reaction has a activation barrier associated to it. V8rik 13:58, 8 June 2007 (UTC)
- You are correct on both counts. In my view a metastable state is one where the activation barrier is so high that nothing happens. The classic example is diamond which is metastable with respect to graphite. In the context of chemical equilibrium it will be better to talk about catalysis and drop the metastable terminology. Petergans 20:09, 15 June 2007 (UTC)
Duplicate images uploaded
Thanks for uploading Image:EAS MetaSubstitutionsvg2.svg. A machine-controlled robot account noticed that you also uploaded the same image under the name Image:EAS MetaSubstitutionsvg.svg. The copy called Image:EAS MetaSubstitutionsvg.svg has been marked for speedy deletion since it is redundant. If this sounds okay to you, there is no need for you to take any action.
This is an automated message- you have not upset or annoyed anyone, and you do not need to respond. In the future, you may save yourself some confusion if you supply a meaningful file name and refer to 'my contributions' to remind yourself exactly which name you chose (file names are case sensitive, including the extension) so that you won't lose track of your uploads. For tips on good file naming, see Misplaced Pages's image use policy. If you have any questions about this notice, or feel that the deletion is inappropriate, please contact User:Staecker, who operates the robot account. Staeckerbot 16:47, 6 June 2007 (UTC)
Duplicate Image:BenzeneFriedelCraftsAlkylation2.png

Hello, this is a message from an automated bot. A tag has been placed on Image:BenzeneFriedelCraftsAlkylation2.png, by Staeckerbot, another Misplaced Pages user, requesting that it be speedily deleted from Misplaced Pages. The tag claims that it should be speedily deleted because Image:BenzeneFriedelCraftsAlkylation2.png is a duplicate of an already existing article, category or image.
To contest the tagging and request that administrators wait before possibly deleting Image:BenzeneFriedelCraftsAlkylation2.png, please affix the template {{hangon}} to the page, and put a note on its talk page. If the article has already been deleted, see the advice and instructions at WP:WMD. Please note, this bot is only informing you of the nomination for speedy deletion, it did not nominate Image:BenzeneFriedelCraftsAlkylation2.png itself. Feel free to leave a message on the bot operator's talk page if you have any questions about this or any problems with this bot. --Android Mouse Bot 2 18:25, 6 June 2007 (UTC)
Lagrange multipliers
If you can give me an e-mail address I will send you a pdf of the part of Chapter 2 from Van Zeggeren and Storey that explains the matter. I have a personal copy of this book, but obviously cannot put the pdf in Wiki. You can find my email on my home page. Just send me a message and I will reply with the pdf. Petergans 21:54, 12 June 2007 (UTC)
Thanks Petergans, for your offer but I am swamped in reading as it is (I am on the galanthamine trail, fasinating stuff!) and math is not my strong suit. My only concern is with readers of the article with interest in chemical equilibrium but lacking proper math skills (or like me in possession of skills a long time ago but since then deteriorated ). I am confident that the three of you (with loom, itub) can work out this section. When the metastability section has had its review I think we have a great article! V8rik 21:37, 13 June 2007 (UTC)
There is a dilemma here: equilibrium chemistry is by its essence quantitative, so equations are needed. I'm not sure that expanding the derivation of expressions is the right approach. Could a verbal explanation of the meaning of an equation be a better way? Petergans 09:26, 15 June 2007 (UTC)
K vs T
I've added comments at talk: equilibrium constant Petergans 09:26, 15 June 2007 (UTC)
Cavicularin
Are you sure about the 15° bond angle in cavicularin? And no, benzene's C-C-C bond angles are 120°, not 0° (I have already corrected this one). Icek 18:01, 15 June 2007 (UTC)
- Thanks Icek, for your intervention, obviously what I meant was the deformation out of planarity, in the cyclophane article I do a better job explaining it. I am confident that your rewrite solved all confusion. V8rik 19:03, 15 June 2007 (UTC)
Deletion
Hi, Just read your User Page on deletions and could not agree with you more!! Some wikipedians seem ruthless when it comes to non-US related info such as International airings of Prison Break. The same instiagor of that deletion deleted details about the international airings of Australian cult show Prisoner (Cell Block H), to which I contributed a great deal to! The deletion policy and process here does seem very very strange indeed and does not really offer a fair process. Anyway, moan over. Topps248 13:03, 16 June 2007 (UTC)
Bent bonds
Sorry about deleting the paragraph from the banana bond article. It was unreferenced and a very unorthodox use of the term that I had never heard before, but now I see it was correct. Doh! --Itub 17:00, 20 June 2007 (UTC)
Hi Itub, thanks for your comment. If you would have tagged the sequences you questioned with a tag, I would have been more than happy to direct them to the Wiberg article. We have had the discussion in Misplaced Pages:Scientific citation guidelines after all. There is no point putting a reference every second word in a sentence! The rant in my user page: User:V8rik#On_deletions that I wrote over a year ago is still valid, battling deletions is almost a daytime job so I am getting a little short-fused. V8rik 19:55, 20 June 2007 (UTC)
License tagging for Image:Bis(1,5-cyclooctadiene)nickel(0).png
Thanks for uploading Image:Bis(1,5-cyclooctadiene)nickel(0).png. Misplaced Pages gets thousands of images uploaded every day, and in order to verify that the images can be legally used on Misplaced Pages, the source and copyright status must be indicated. Images need to have an image tag applied to the image description page indicating the copyright status of the image. This uniform and easy-to-understand method of indicating the license status allows potential re-users of the images to know what they are allowed to do with the images.
For more information on using images, see the following pages:
This is an automated notice by OrphanBot. If you need help on selecting a tag to use, or in adding the tag to the image description, feel free to post a message at Misplaced Pages:Media copyright questions. 21:07, 24 June 2007 (UTC)
Image:Dibenzalacetonesynth.png
Hi V8rik. Can you please move that image to Commons, so that it can be used in other wikipedias? Thanks. -- 212.41.66.201 15:12, 1 July 2007 (UTC)
Non mixed edge and face capping
Well your re-edits baffle me, because I cannot find such a structure in the Welch-Long review. I am pretty familiar with the area, having published on such clusters, and would certainly welcome a reference in support of the structure you depict. It is possible that I missed such a paper given the ocean of literature. But a framework that is not Oh symmetry with respect to the location of the inner ligands would be a treat to me vs. the usual M6X18 motif. CAS depicts Nb6F15 (RN# 12140-31-7) as having no face-bridging halides, in contrast to your statement. Could identify a publication that describes an octahedral cluster with a mixture of both edge and face-capping ligands - Long's review does not. Looking forward to your help or clarification.--Smokefoot 22:34, 2 July 2007 (UTC)
- Hi Smokefoot, you being SO impatient!. You really should allow people to check there own literature. By chance (I found the reference (1) on the internet, that saves me a trip to the library) I was able to do this so i will make the required changes. V8rik 18:49, 3 July 2007 (UTC)
Galanthamine total synthesis
A great article, but who cares? Only a very few biochemists in the whole world. Does it need so much room on Misplaced Pages? A couple of references in the main article should do it.Mwinog2777 05:39, 11 July 2007 (UTC)
I do not think any biochemist would care, I do hope organic chemists will care. I see from your edit history that you do not have a science background so that explains a lot. galanthamine is important because it is one of those complex biomolecules that can be made either synthetically or from a natural source. The main reason I have included several total syntheses of biomolecules of this type is because the hundreds of chemical reactions, chemical reagents and concepts described in Wiki are ultimately used in the synthesis of this kind of molecule. I am completely amazed by your suggestion that the article should be limited to references. The one reason I write for Wiki is that too much scientific content is locked away behind subscriptions while I think it should be accessible to the public for free. Apparently you have easy access to all the literature cited but you should understand that on a global scale you are privileged. I am also not aware that the Wiki organization is in shortage of server space but i am sure that when that time is there Jimbo will not delegate the scrapping of articles to you. V8rik 16:42, 11 July 2007 (UTC)
On the contrary, I have a science background. If not, it is unlikely I would have come across the totally obscure over-written section on galantamine. Among my other interests, I am a neurologist, and prescribe galantamine on a regular basis. Also, I helped my daughter get a "B" in honors biology this last semester. I don't scrap articles, I try to make them better. Mwinog2777 07:15, 13 July 2007 (UTC)
- I am glad to read that it is not your intention to have the article scrapped. I think I can explain why you feel the article is overwritten. In writing these articles (I am afraid you haven't seen the taxol total synthesis pages yet) i was inspired by the book Classics in Total Synthesis: Targets, Strategies, Methods K. C. Nicolaou, E. J. Sorensen. It is a real classic in org chem and I basically followed their lead with one exception. The book is intended for experienced organic chemists and a lot of knowledge is assumed. Now in Wiki we cannot assume any prior knowledge and therefore in my texts each synthetic step is explained with as much detail as needed for the less experienced reader. I think I am providing a service doing this but I do realize I am not writing a Tom Clancy thriller. By the way i was wondering since galanthamine according to my sources is worth 50000 dollar per kilo would you be able to tell me how much a galanthamine course costs for a patient? And is there already a synthetic galanthamine on the market? and if there is already a bio-derived version are the clinical trials to be repeated? Congratulations on the B! V8rik 20:40, 13 July 2007 (UTC)
Keep up the good work!
Just dropped by to say that at least I am happy to see all the work you put on Misplaced Pages, especially adding total syntheses. For example, I wanted for some time to add the synthesis of cubane but kept postponing it because I'm really lazy when it comes to drawing structures, and was delighted to see that you added it. It may be a subject that some people consider esoteric, but that's the advantage of Misplaced Pages not being paper; it can aim not only for the scope of a general encyclopedia, but also to the union of all specialized encyclopedias as well. --Itub 08:18, 20 July 2007 (UTC)
- Thanks! V8rik 16:34, 20 July 2007 (UTC)
Imidazolidinone organocatalysis section expansion
Is there any chance you'd be able to expand the section? There's not much of a description of the process or mechanism (if different) there. Thanks :-) ♥♥ ΜÏΠЄSΓRΘΠ€ ♥♥ 23:13, 21 July 2007 (UTC)
- Hi Minestrone Soup, I have clarified the section and linked to the page where its use is demonstrated but I have no urgent expansion plans. V8rik 20:05, 22 July 2007 (UTC)
Article idea
I noticed you created the dodecahedrane article, but we still don't have a pagodane article. Just an idea in case you find yourself one day with nothing to do. ;-) --Itub 08:53, 24 July 2007 (UTC)
- Absolutely, Misplaced Pages is not complete with an entry on pagodane V8rik 21:36, 24 July 2007 (UTC)
File:Triquinacene.png

Thank you for uploading images/media such as Image:Triquinacene.png to Misplaced Pages! There is however another Wikimedia foundation project called Wikimedia Commons, a central media repository for all free media. In the future, please consider creating an account and uploading media there instead. That way, all the other language Wikipedias can use them too, as well as our many sister projects. This will also allow our visitors to search for, view and use our media in one central location. If you wish to move previous uploads to Commons, see Misplaced Pages:Moving images to the Commons. Please note that non-free content, such as images claimed as fair use, cannot be uploaded to the Wikimedia Commons. Help us spread the word about Commons by informing other users, and please continue uploading!
In addition, the image could be more effectively rendered as a vector image.
Sfan00 IMG 21:10, 4 August 2007 (UTC)
Polyurethane
In the Polyurethane article, regarding reaction being between two polymers, well, they can, PMDI + a polyether polyol is such, but that is splitting hairs. The use of polyisocyanate and polyhydroxyl was meant to indicate the molecules had more than one isocyanate group or more than one alcohol group. I struggled with this mightily, as it always seems weird to me to call a polymer a monomer, as in a polyether or polyester polyol. I went into more detail on this in the chemistry section. Regardless, I can accept the use of monomer in the intro as being somewhat less confusing. Best Regards, P Cottontail 20:32, 22 August 2007 (UTC)
- Thanks for your comment: my revision may appear overly technical but the existing one was simply confusing. The simplest of PU's are made from a diisocyanate and a diol, no polymers involved. I would also mention monomer somewhere in the lead. V8rik 20:38, 22 August 2007 (UTC)
Thank you for your comments as well. I'd love to see this as a featured article. Any thought on converting the reaction diagram .png's (at 96 pix/in) to .svg's? Cheers, P Cottontail 20:45, 22 August 2007 (UTC)
- I know editors who are in the know on png - svg conversions, i will ask one of them V8rik 20:57, 22 August 2007 (UTC)
Schiff test
Your picture of Schiff test ist wrong. I took it out of the article. As I have not enough time right now I would please you to make a better version. And after that you could insert it into the german and french article to.
THANKS (lictuel from the german wiki) —The preceding unsigned comment was added by 141.53.203.95 (talk) 05:34, August 23, 2007 (UTC)
- I have restored the image for now because I feel that although the image indeed contains errors the main message contained in the image is valid. I also prefer discussion prior to deletions. I have researched the matter and I will make new edits. The literature I have consulted are at odds with the content of the Regensburg website though so lets not include that one. V8rik 19:41, 24 August 2007 (UTC)
List of chemical compounds with unusual names
I have to say I like the new introduction. I'm not a chemist but even I can understand it! Thin Arthur 06:54, 30 August 2007 (UTC)
 HCO3 + H by bringing the reactants into close proximity. The backward reaction is fast anyway. So, the enzyme speeds up only the forward reaction so that equilibrium is reached quickly. The backward reaction has a quite different reaction profile. For one thing, the ions carry opposite charges so they will be easiliy attracted to each other, whereas CO2 is hydrophobic.
HCO3 + H by bringing the reactants into close proximity. The backward reaction is fast anyway. So, the enzyme speeds up only the forward reaction so that equilibrium is reached quickly. The backward reaction has a quite different reaction profile. For one thing, the ions carry opposite charges so they will be easiliy attracted to each other, whereas CO2 is hydrophobic.
 . N2 can easily be generated in the gas phase once there are radicals flying around. Certainly the reaction
. N2 can easily be generated in the gas phase once there are radicals flying around. Certainly the reaction  does not need a catalyst. Is there a detailed kinetic study of ammonia dissociation in the literature?
does not need a catalyst. Is there a detailed kinetic study of ammonia dissociation in the literature?