This is an old revision of this page, as edited by 130.102.0.176 (talk) at 22:26, 4 October 2005. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 22:26, 4 October 2005 by 130.102.0.176 (talk)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| IUPAC chemical name | |
| CAS number 59729-33-8 |
ATC code N06AB04 |
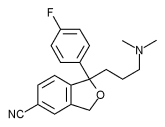
| Chemical formula | C20H22BrFN2O |
| Molecular weight | 405.35 |
| Bioavailability | ? |
| Metabolism | CYP3A4 and CYP2C19 Predominantly |
| Elimination half-life | ? |
| Excretion | ? |
| Pregnancy category | ? |
| Legal status | Free to Market; patent expired |
| Routes of administration | Oral |
Citalopram or nitalapram is an antidepressant drug used to treat the depression associated with mood disorders. It is also used on occasion in the treatment of body dysmorphic disorder and anxiety.
Citalopram is one of a class of drugs known as selective serotonin reuptake inhibitors (SSRIs). It is sold under the brand names Celexa™ (US), Cipramil™ (Europe and Australia) and Talohexane (Australia).
Citalopram was originally created by the pharmaceutical company Lundbeck although the patent for it expired in 2003, allowing other companies to legally produce generic versions.
Lundbeck has recently released a new SSRI drug called escitalopram oxalate (also known as Cipralex or Lexapro) derived from the citalopram molecule.
In the United States, the drug is marketed under the name Celexa™ by Forest Laboratories, Inc.
Reported side effects
Over 10% of patients
- Feeling sick or tired
- Drowsiness
- Dry mouth
- Sweating
- Trembling
- Headache
- Dizziness
- Difficulty sleeping
- Feeling agitated or nervous
- Constipation or diarrhoea
- Blurred vision
- Missed heartbeats
- Feeling of weakness
- Difficulty ejaculating
Over 1% of patients
- Itchiness
- Rashes
- Migraine
- Strange dreams
- Changes in taste
- Decreased libido or impotence
- Failure to orgasm
- Painful menstruation
- Tingling in fingers or toes
- Loss of memory or concentration
- Changes in appetite (increase or lack of)
- Mood changes
- Anxiety
- Confusion
- Yawning
- Indigestion
- Vomiting
- Stomach ache
- Flatulence
- Increased saliva
- Change in weight
- Dizziness on standing up
- Fast heartbeat
- Changes in blood pressure
- Runny nose
- Sinusitis
- Changes in passing urine
Rare (under 1% of patients)
- Muscle pain
- Convulsions
- Increased libido
- Coughing
- Abnormal movement of the face or body
- Ringing in the ears
- Mood changes
- Slowing of the heartbeat
- Photosensitivity
- Allergic reactions
- Fainting
- Attemptive suicide
- Abrupt change in musical preference
Other side effects
Occasionally, panic attacks, thoughts of suicide or self-harm may occur or increase in the first few weeks, before the antidepressant effect starts.
Other occasional effects include leaking of milk from the breasts, changes in heart rhythm, swelling of the skin, joint pain or severe allergic reactions.
External links
Pharmacological information and treatment study information:
- http://www.biopsychiatry.com/citalopram.html
- Celexa / Citalopram Fact Sheet
- FDA patient fact sheet on Citalopram hydrobromide
Lunbeck's official websites for citalopram under the trade name Cipramil:
Forest's official websites for citalopram under the trade name Celexa:
Categories: