This is an old revision of this page, as edited by Citation bot 1 (talk | contribs) at 16:38, 3 August 2010 (Citations: Tweaked: url. You can use this bot yourself! Report bugs here.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 16:38, 3 August 2010 by Citation bot 1 (talk | contribs) (Citations: Tweaked: url. You can use this bot yourself! Report bugs here.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
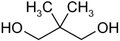
| IUPAC name 2,2-Dimethyl-1,3-propanediol | |||
| Identifiers | |||
| CAS Number | |||
| ECHA InfoCard | 100.004.347 | ||
| CompTox Dashboard (EPA) | |||
| Properties | |||
| Chemical formula | C5H12O2 | ||
| Molar mass | 104.148 g/mol | ||
| Melting point | 129.13°C | ||
| Boiling point | 208°C | ||
| Solubility in water | soluble in water | ||
| Solubility | soluble in benzene, chloroform, very soluble in ethanol, diethyl ether | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH298) |
-551.2 kJ•mol | ||
| Hazards | |||
| Flash point | 129°C | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Neopentyl glycol (IUPAC name 2,2-dimethyl-1,3-propanediol) is an organic chemical compound. It is used in the sythesis of polyesters, paints, lubricants, and plasticizers. When used in the manufacture of polyesters, it enhances the product's stability toward heat, light, and water.
Reactions
Neopentyl glycol is synthesized industrially by the aldol reaction of formaldehyde and isobutyraldehyde. This creates the intermediate hydroxypivaldehyde, which can be converted to neopentyl glycol with either excess formaldehyde or catalytic hydrogenation of the aldehyde group to an alcohol group.
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3‑228, 5‑42, 16‑22, ISBN 0849305942
- Weissermel, Klaus; Arpe, Hans-Jürgen; Lindley, Charlet R. (2003), Industrial Organic Chemistry (4 ed.), Wiley-VCH, pp. 214–215, ISBN 9783527305780, retrieved 2009-07-20