This is an old revision of this page, as edited by Khareldn (talk | contribs) at 23:43, 5 October 2010. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:43, 5 October 2010 by Khareldn (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Orotic acid" – news · newspapers · books · scholar · JSTOR (October 2007) (Learn how and when to remove this message) |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.563 |
| Chemical and physical data | |
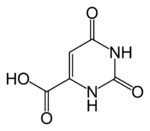
| Formula | C5H4N2O4 |
| Molar mass | 156.10 g/mol g·mol |
| (verify) | |
Orotic acid is a heterocyclic compound and an acid; it is also known as pyrimidinecarboxylic acid. It was once believed to be part of the vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin but is instead manufactured in the body by intestinal flora.
Its salts, known as orotates, are sometimes used as mineral carriers in some dietary supplements, to increase their bioavailability. Lithium orotate is the most frequently used in this manner.
Synthesis of Orotate
Dihydroorotate is synthesized to Orotic Acid by Dihydroorate Dehydrogenase, where it later combines with PRPP (Phosphoribosyl Pyrophosphate) to form Orotidylate (OMP). A distinguishing characteristic of pyrimidine synthesis is that the pyrimidine ring is fully synthesized before being attached to the ribose sugar, whereas purine synthesis happens by building the base directly on the sugar.
Orotic acid diseases
A buildup of orotic acid can lead to orotic aciduria. It may be a symptom of an increased ammonia load due to a metabolic disorder, such as a urea cycle disorder.
In ornithine transcarbamoylase deficiency, a disorder of the urea cycle, excess carbamoyl phosphate is converted into orotic acid. This typically leads to increased urinary orotic acid excretion, because the orotic acid is not being properly utilized and must be eliminated.
Orotic acid can be mutagenic (causes mutations) in mammalian somatic cells. It is also mutagenic for bacteria and or yeast.
See also
References
- "Vitamin B13 (Orotic Acid)".
- Husebye E, Skar V, Høverstad T, Melby K (1992). "Fasting hypochlorhydria with gram positive gastric flora is highly prevalent in healthy old people". Gut. 33: 1331–1337. doi:10.1136/gut.33.10.1331.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Greenbaum SB (1954). "Orotic acid antagonist: "6-Uracilsulfonic Acid, a Sulronic Acid Analog of Orotic Acid". J. Am. Chem. Soc. 76 (23): 6052–6054.
- Lippincott. Biochemistry 4th Edition. 2008
External links
- Orotic+Acid at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview at ggc.org
- Overview at greatvistachemicals.com
- Overview of Potential Metabolic Antagonists
| Nucleotide metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| purine metabolism |
| ||||||||||
| pyrimidine metabolism |
| ||||||||||
This pharmacology-related article is a stub. You can help Misplaced Pages by expanding it. |