This is an old revision of this page, as edited by Beetstra (talk | contribs) at 14:23, 12 December 2010 (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:23, 12 December 2010 by Beetstra (talk | contribs) (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)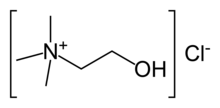
| |
| Names | |
|---|---|
| IUPAC name 2-hydroxy-N,N,N-trimethylethanaminium chloride OR (2-hydroxyethyl)trimethylammonium chloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.000.596 |
| E number | E1001(iii) (additional chemicals) |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H14ClNO |
| Molar mass | 139.62 g·mol |
| Appearance | White or deliquescent crystals |
| Melting point | 302 °C (decomposes) |
| Solubility in water | very soluble (>650 g/l) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Choline chloride is an organic compound and a quaternary ammonium salt. It has a choline cation with chloride anion. Alternative names are hepacholine, biocolina and lipotril.
Synthesis
In the laboratory choline can be prepared by methylation of dimethylethanolamine with methyl chloride.
In the industrial Davy Process Technology route choline chloride is produced from ethylene oxide, hydrochloric acid, and trimethylamine, or from the pre-formed salt:
Applications
Choline chloride is mass produced and is an important additive in feed especially for chicken where it accelerates growth. With urea it forms a deep eutectic solvent. Other commercial choline salts are choline hydroxide and choline bitartrate. In foodstuffs the compound is often present as phosphatidylcholine.
References
- "Chemical Safety Information from Intergovernmental Organizations - Choline Chloride" (PDF).
- Davy Process Technology
- "Choline chloride" (PDF). Screening Information Data Set (SIDS) for High Production Volume Chemicals. IPCS INCHEM.