This is an old revision of this page, as edited by Kupirijo (talk | contribs) at 02:18, 15 December 2010 (Category:Benzenesulfonates). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 02:18, 15 December 2010 by Kupirijo (talk | contribs) (Category:Benzenesulfonates)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)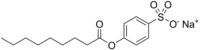
| |
| Names | |
|---|---|
| IUPAC name Sodium 4-nonanoyloxybenzenesulfonate | |
| Other names 4-Sulfophenyl nonanoate sodium salt; Sodium p-nonanoyloxybenzenesulfonate; p-(Nonanoyloxy)benzenesulfonic acid sodium salt; p-Sodiosulfophenyl nonanoate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | NOBS |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H21NaO5S |
| Molar mass | 336.38 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sodium nonanoyloxybenzenesulfonate (NOBS) is an important component of detergents and bleaches. It is known as a bleaching activator for active oxygen sources, allowing formulas containing hydrogen peroxide releasing chemicals (specifically the sodium perborate, sodium percarbonate, sodium perphosphate, sodium persulfate, and urea peroxide. Unlike tetraacetylethylenediamine, NOBS can be used in a much lower temperature.
References
- Kuzel, P.; Lieser, T. (1990). "Bleach systems". Tenside, Surfactants, Detergents. 27 (1): 23–8.
{{cite journal}}: CS1 maint: multiple names: authors list (link)