This is an old revision of this page, as edited by Beetstra (talk | contribs) at 13:02, 17 February 2011 (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:02, 17 February 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Dimethylacetamide | |||
| Other names DMAc, DMA, acetic acid-dimethylamide, N,N-dimethylacetamide, acetyldimethylamine | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.389 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H9NO | ||
| Molar mass | 87.122 g·mol | ||
| Appearance | Colorless liquid with faint ammonia odor | ||
| Density | 0.94 g/cm | ||
| Melting point | −20 °C (−4 °F; 253 K) | ||
| Boiling point | 164-166 °C | ||
| Viscosity | 1.956 cP @ 25 °C 1.279 cP @ 50 °C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Toxic (T) | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 70 °C | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
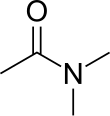
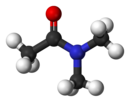
Dimethylacetamide is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water miscible, high boiling liquid is commonly used as a polar solvent in organic chemistry. DMAc is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.
The chemical reactions of dimethylacetamide are typical of N,N-disubstituted amides. It will hydrolyze in the presence of acids:
- CH3CON(CH3)2 + H2O + HCl → CH3COOH + (CH3)2NH2Cl
Dimethylacetamide is useful as a medium for strong bases such as sodium hydroxide. Dimethylacetamide is commonly used as a solvent for fibers or in the adhesive industry. It is also employed in the production of pharmaceuticals and plasticizers as a reaction medium.
References
- S. Zen and E. Kaji (1988). "Dimethyl nitrosuccinate". Organic Syntheses; Collected Volumes, vol. 6, p. 503.