This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 18:05, 25 February 2011 (Updating {{drugbox}} (changes to watched fields - updated 'UNII_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_Pharmacolog). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 18:05, 25 February 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (changes to watched fields - updated 'UNII_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_Pharmacolog)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound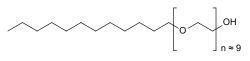 | |
| Clinical data | |
|---|---|
| Other names |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.019.351 |
| Chemical and physical data | |
| Formula | C30H62O10 |
| Molar mass | ~600 g/mol (average) g·mol |
| (verify) | |
Polidocanol is a local anaesthetic and antipruritic component of ointments and bath additives. It relieves itching caused for example by dry skin conditions such as eczema.
The substance is also used as a sclerosant, an irritant injected to treat varicose veins, under the trade names Asclera and Aethoxysklerol. Polidocanol causes fibrosis inside varicose veins, occluding the lumen of the vessel, and reducing the appearance of the varicosity.
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue.
References
- "E45 itch relief cream". netdoctor.co.uk. Retrieved 2007-07-12.
- Sclerotherapy, Laurence Z Rosenberg, MD, eMedicine.com
- Facts and Companies: Varicose Vein Treatment Approved
- "Asclera Full Prescribing Information in Drug Reference Encyclopedia". Retrieved 2010-04-11.
| Vasoprotectives (C05) | |||||||
|---|---|---|---|---|---|---|---|
| Antihemorrhoidals for topical use |
| ||||||
| Antivaricose therapy |
| ||||||
| Capillary stabilising agents |
| ||||||
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |
This dermatologic drug article is a stub. You can help Misplaced Pages by expanding it. |