This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 17:58, 8 March 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 17:58, 8 March 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Perylene | |||
| Other names peri-Dinaphthalene; Perilene; Dibenzanthracene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ECHA InfoCard | 100.005.365 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C20H12 | ||
| Molar mass | 252.316 g·mol | ||
| Appearance | Brown solid | ||
| Melting point | 276-279 °C | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
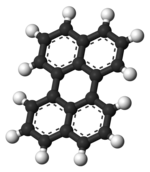
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes.
Emission
Perylene displays blue fluorescence. It is used as a blue-emitting dopant material in OLEDs, either pure or substituted. Perylene can be also used as an organic photoconductor. It has an absorption maximum at 434 nm, and as with all polycyclic aromatic compounds, low water solubility (1.2 x 10 mmol/L). Perylene has a molar absorptivity of 38,500 Mcm at 435.75 nm (about 6.5 μg is sufficient for absorbence of 1).
-
 Perylene dissolved in dichloromethane exposed to Long Wave UV Light
Perylene dissolved in dichloromethane exposed to Long Wave UV Light
-
 Perylene dissolved in dichloromethane exposed to Short Wave UV Light
Perylene dissolved in dichloromethane exposed to Short Wave UV Light
Structure
The perylene molecule consists of two naphthalene molecules connected by a carbon-carbon bond at the 1 and 8 positions on both molecules. All of the carbon atoms in perylene are sp hybridized. When drawing the structure of perylene, it is important not to represent the center ring as a fifth benzene ring. By doing so, this would depict two of the carbons as sp hybridized and fail to reflect the aromaticity of part of the molecule, and therefore its ability to fluoresce. The structure of perylene has been extensively studied by X-ray crystallography.
References
- Perylene at Sigma-Aldrich
- Donaldson, D. M.; Robertson, J. M.; White, J. G. (1953). First Page (JSTOR) "The crystal and molecular structure of perylene". Proc. R. Soc. Lond. A Math. Phys. Sci. 220 (1142): 311–321. doi:10.1098/rspa.1953.0189.
{{cite journal}}: Check|url=value (help)CS1 maint: multiple names: authors list (link)
| Polycyclic aromatic hydrocarbons | |
|---|---|
| 2 rings | |
| 3 rings | |
| 4 rings | |
| 5 rings | |
| 6 rings | |
| 7+ rings | |
| General classes | |