This is an old revision of this page, as edited by Azuidema (talk | contribs) at 14:59, 22 March 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:59, 22 March 2011 by Azuidema (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound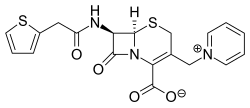 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.048 |
| Chemical and physical data | |
| Formula | C19H17N3O4S2 |
| Molar mass | 415.486 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Cefaloridine (or cephaloridine) is first generation semisynthetic derivative of Cephalosporin C. It is unique among cephalosporins in that it exists as a zwitterion.
History
Since the discovery of cephalosporins P, N and C in 1948 there have been many studies describing the antibiotic action of cephalosporins and the possibility to synthesize derivatives. Hydrolysis of cephalosporin C, isolation of 7-aminocephalosporanic acid and the addition of side chains opened the possibility to produce various semi-synthetic cephalosporins. In 1962, cephalothin and cephaloridine were introduced.
Cephaloridine was temporary popular because it was better tolerated intramuscular and attained in higher and more sustained levels in blood than cephalothin. However, it binds to proteins to a much lesser extent than cephalothin. Because it is also poorly absorbed after oral administration the use of this drug for humans declined rapidly, especially since the second generation of cephalosporins was introduced in the 1970s. Today it is more commonly used in veterinary practice to treat mild to severe bacterial infections caused by penicillin resistant and penicillin sensitive Staphylococcus aureus, Escherichia coli, Streptococcus pyogenes, Streptococcus pneumoniae, Bacillus sutbtilis, Klebsiella, Clostridium diptheriae, Salmonella and Shigella. Interest in studying cephalosporins was brought about by some unusual properties of cephaloridine. This antibiotic stands in sharp contrast to various other cephalosporins and to the structurally related penicillins in undergoing little or no net secretion by the mammalian kidney. Cephaloridine is, however, highly cytotoxic to the proximal renal tubule, the segment of the nephron responsible for the secretion of organic anions, including para-am-minohippurate (PAH), as well as the various penicillin and cephalosporin antibiotics. The cytotoxicity of cephaloridine is completely prevented by probenecid and several other inhibitors of organic anion transport, including the nearly nontoxic cephalosporin cephalothin.
Structure & reactivity
Cephaloridine is a cephalosporin compound with pyridinium-1-ylmethyl and 2-thienylacetamido side groups. The molecular nucleus, of which all cephalosporins are derivatives, is A3-7-aminocephalosporanic acid. Conformations around the β-lactam rings are quite similar to the molecular nucleus of penicillin, while those at the carboxyl group exocyclic to the dihydrothiazine and thiazolidine rings respectively are different.
Synthesis
Cephaloridine can be synthesised from Cephalotin and pyridine by deacetylation. This can be done by heating an aquous mixture of cephalotin, thiocyanate, pyridine and phosphoric acid for several hours. After cooling, diluting with water, and adjusting the pH with mineral acid, cephaloridine thiocyanate salt precipitates. This can be purified and converted to cephaloridine by pH adjustment or by interaction with ion-exchange resin.
Kinetics
Absorption
Cephaloridine is after intramuscular injection easy absorbed and it is poorly absorbed from the gastrointestinal tract.
Distribution
The minor pathway of elimination is biliary excretion. When the blood serum concentration is 24µg/ml, the corresponding biliary concentration is 10µg/ml. In de spinal fluid the concentration of Cephaloridine is 6-12% of the concentration in de blood and serum. Cephaloridine is distributed well into the liver, stomach wall, lung and spleen and is also found in fresh wounds one hour after injection. The concentration in the wound will decrease as the wound age increase. However, the drug is poorly penetrated into the cerebrospinal fluid and is found in a much smaller amount in the cerebral cortex.
Adverse effects
Toxicity
Cephaloridine can cause kidney damage in humans since it is actively taken up from the blood by the proximal tubular cells via an organic anion transporter (OAT) in the basolateral membrane. Organic anions are secreted through the proximal tubular cells via unidirectional transcellular transport. The organic anions are taken up from the blood into the cells across the basolateral membrane and extruded across the brush border membrane into the tubular fluid. Cephaloridine is a substrate for OAT-1 and thus can be transported into the the proximal tubular cells, which form the renal cortex. The drugs, however, cannot move readily across the luminal membrane since it is a zwitterion. The cationic group (pyridinium ring) of the compound probably inhibits the efflux through the membrane. This results in an accumulation of cephaloridine in the renal cortex of the kidney, causing damage and necrosis of the S2 segment of the tubule.
Metabolism
Chephaloridine is excreted in the urine without undergoing metabolism. It inhibits organic ion transport in the kidney. This proces is preceded by the lipid peroxidation. Therafter, probably a combination of events, such as formation of a reactive intermediate, a free radical and stimulation of lipid peroxidation, lead to peroxidative damage to cell membranes and mitochondria. It is not yet clear whether metabolic activation by cytochromes P-450, chemical rearrangements, reductive activation or all these actions are involved.
The hypotheses made about the mechanism of action causing the toxiciy of cephaloridine are:
- Reactive metabolites are formed by cytochromes P-450 or emerge from destabilization of the β –lactam ring. Metabolic activation of the drugs might take place via cytochromes P-450, producing reactive metabolites. This hypothesis is formed because some inhibitors of CYTP450 decrease the toxicity and some inducers of the monooxygenases increase toxicity. It could also be possible that a reactive intermediate is formed since the β -lactam ring is unstable. The pyridinium side-group of cephaloridine has unstable bonds to the core of the compound (in comparation with other cephalosporins). When this side-group leaves, the β –lactam ring is destabalized by intramolecular electron shifts. Thus, the leaving group creates a reactive product.
- Both lipid peroxidation and oxidative stress can cause membrane damage. Lipid peroxidation and oxidative stress take place since lipid peroxidation products, such as malondialdehyde, have been detected. Reduced glutathione (GSH) and NADPH are both depleted. Consequently, GSSG cannot be reduced to GSH. This leads to an increased toxicity since oxidative stress cannot be reduced. In addition, nephrotoxicity is augmented by deficiency of selenium or tocopherol. The pyridinium side-group interacts with the reduced NADP in a redox cycle. It has been suggested that superoxide anion radical and hydroxyl radical may be formed and that lipid peroxidation could be responsible for the toxicity of cephaloridine.
- Damage to the mitochondria and intracellular respiratory processes and reduced mitochondrial respiration can cause nephrotoxicity. The previous mentioned damages have been detected after exposure to cephalosporins. β-lactam antibiotics injure mitochondria by an attack on the metabolic substrate carriers of the inner membrane. Respiratory toxicity is caused by inactivation of mitochondrial anion substrate carriers.
Symptoms of kidney damage caused by cephaloridine
Some symptoms caused by cephaloridine are: asymptomatic, enzymuria, proteinuria, tubular necrosis, increased urea level in blood, anemia, increased hydrogen ion level in blood, fatigue, increased blood pressure, increased blood electrolyte level, kidney dysfunction, kidney damage, impaired body water balance and impaired electrolyte balance.
Complications caused by cephaloridine
Complications caused by the use of cephaloridine include seizures, coma, chronic kidney failure, acute kidney failure and death.
References
- ^ Mason, I.S, Kietzmann, M., Cephalosporins-pharmalogical basis of clinical use in veterinary dermatology, Veterinary Dermatology 1999, 10, 187-192
- R.K. CHAUDHARY AND A.K. SRIVASTAVA, Disposition and dosage regimen of cephaloridine in calves, Vezerkny Research Communications, 13 (1989) 325-329. http://www.springerlink.com/content/l67g7035w9113317/fulltext.pdf
- Bruce M Tune and Doris Fravert, Mechanisms of cephalosporin nephrotoxicity: A comparison of cephaloridine and cephaloglycin, Kidney International (1980) 18, 591–600; doi:10.1038/ki.1980.177 http://www.nature.com/ki/journal/v18/n5/pdf/ki1980177a.pdf
- R. M. Sweet and L. F. Dahl, THE STRUCTURE OF CEPHALORIDINE HYDROCHLORIDE MONOHYDRATE, BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS 1969 Vol. 34, No. 1
- Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1120
- ^ Michio Takeda, Akihiro Tojo, Takashi Sekine, Makoto Hosoyamada, Yoshikatsu Kanai and Hitoshi Endou, Role of organic anion transporter 1 (OAT1) in cephaloridine (CER)-induced nephrotoxicity, Kidney International (1999), http://www.nature.com/ki/journal/v56/n6/full/4491152a.html
- ^ John A. Timbrell, Principles of Biochemical Toxicology, Informa Healthcare USA Inc., 2009, p 332-335
- Robert W. Schrier, Diseases Of The Kidney And The Urinary Tract, Lippincott Williams & Wilkins, 2007, p 1041 http://books.google.nl/books?id=ERqtOZMAiw0C&pg=PA1041&lpg=PA1041&dq=Cephaloridine+reactivity&source=bl&ots=tZiGHvQKvZ&sig=NBOP6rdDIOHJZ0bovIuCwZBkqVQ&hl=nl&ei=mGpeTcTeLs-UOsPGyekN&sa=X&oi=book_result&ct=result&resnum=1&ved=0CBYQ6AEwADgK#v=onepage&q=Cephaloridine%20reactivity&f=false
- Marvin Turck, Cephalosporins and Related Antibiotics: An Overview, REVIEWS OF INFECTIOUS DISEASES • VOL. 4, SUPPLEMENT • SEPTEMBER-OCTOBER 1982, © 1982 by The University of Chicago. http://cid.oxfordjournals.org/content/4/Supplement_2/S281.full.pdf
- ^ Bruce M. Tune, Nephrotoxicity of beta-lactam antibiotics: mechanisms and strategies for prevention, Pediatr Nephrol (1997) 11: 768±772, http://www.springerlink.com/content/3q23m092mlpn7rv5/
- ^ http://www.wrongdiagnosis.com/k/kidney_damage_cephaloridine/intro.htm