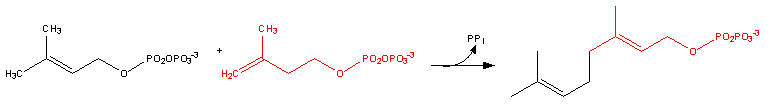This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 09:25, 18 April 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:25, 18 April 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Farnesyl pyrophosphate" – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this message) |

| |
| |
| Identifiers | |
|---|---|
| CAS Number | |
| MeSH | farnesyl+pyrophosphate |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | C15H28O7P2 |
| Molar mass | 382.326 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Farnesyl pyrophosphate (FPP) is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes, terpenoids, and sterols.
It is the immediate precursor of squalene (via the enzyme squalene synthase), dehydrodolichol diphosphate (a precursor of dolichol), and geranylgeranyl pyrophosphate (GGPP).
Biosynthesis
Farnesyl pyrophosphate synthase (a prenyl transferase) catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate.
- Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrophosphate:
- Geranyl pyrophosphate itself reacts with 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate
Regulation
The above reactions are inhibited by bisphosphonates (used for osteoporosis).
Related compounds
| Cholesterol and steroid metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonate pathway |
| ||||||||||
| Non-mevalonate pathway | |||||||||||
| To Cholesterol | |||||||||||
| From Cholesterol to Steroid hormones |
| ||||||||||
| Nonhuman |
| ||||||||||
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |