This is an old revision of this page, as edited by Why Not A Duck (talk | contribs) at 23:35, 20 April 2011 (Undid revision 425000266 by 14.139.160.2 (talk) -- would its aromaticity actually depend on temperature? seems dubious. source or explanation?). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:35, 20 April 2011 by Why Not A Duck (talk | contribs) (Undid revision 425000266 by 14.139.160.2 (talk) -- would its aromaticity actually depend on temperature? seems dubious. source or explanation?)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
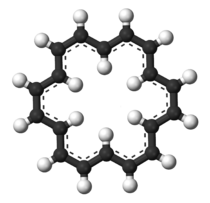
| |
| Identifiers | |
|---|---|
| CAS Number | |
InChI
| |
| Properties | |
| Chemical formula | C18H18 |
| Molar mass | 234.342 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cyclooctadecanonaene or annulene is an annulene with chemical formula C18H18. This hydrocarbon obeys Hückel's rule and is therefore an aromatic compound. The compound was first synthesised by Franz Sondheimer. The original synthesis started by the Eglinton reaction of the di-alkyne 1,5-hexadiyne with copper(II) acetate in pyridine to give the trimer, followed by deprotonation and isomerization with potassium tert-butoxide in tert-butanol and was concluded with hydrogen organic reduction with the Lindlar catalyst.
References
- In the literature and some internet references Sondheimer is misspelled as Sandheimer.
- Sondheimer, F., Wolovsky, R. and Amiel, Y. (1962). "Unsaturated Macrocyclic Compounds. XXIII. The Synthesis of the Fully Conjugated Macrocyclic Polyenes Cyclooctadecanonaene (Annulene), Cyclotetracosadodecaene (Annulene), and Cyclotriacontapentadecaene (Annulene)". J. Am. Chem. Soc. 68 (2): 274–284. doi:10.1021/ja00861a030.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - K. Stöckel and F. Sondheimer (1988). "Annulene". Organic Syntheses; Collected Volumes, vol. 6, p. 68.