This is an old revision of this page, as edited by Sheac (talk | contribs) at 04:53, 17 May 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 04:53, 17 May 2011 by Sheac (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)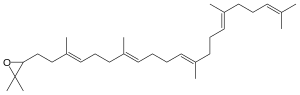
| |
| Names | |
|---|---|
| IUPAC name 2,2-Dimethyl-3-oxirane | |
| Other names
Squalene oxide 2,3-Squalene oxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| MeSH | 2,3-oxidosqualene |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C30H50O |
| Molar mass | 426.717 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2,3-(S) Oxidosqualene ((S)-2,3-epoxysqualene) is an intermediate in the synthesis of the membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed from squalene by squalene monooxygenase. 2,3 oxidosqualene is the substrate of various oxidosqualene cyclases, including lanosterol synthase, which produces lanosterol, a precursor to cholesterol.
2,3-(R) Oxidosqualene is an inhibitor of lanosterol synthase.
References
- Abe I. (2007). "Enzymatic synthesis of cyclic triterpenes". Natural Products Reports. 24 (6): 1311–31. doi:10.1039/b616857b. PMID 18033581.
| Cholesterol and steroid metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonate pathway |
| ||||||||||
| Non-mevalonate pathway | |||||||||||
| To Cholesterol | |||||||||||
| From Cholesterol to Steroid hormones |
| ||||||||||
| Nonhuman |
| ||||||||||
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |