This is an old revision of this page, as edited by Chemicalinterest (talk | contribs) at 13:00, 24 May 2011 (→Reactions). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:00, 24 May 2011 by Chemicalinterest (talk | contribs) (→Reactions)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)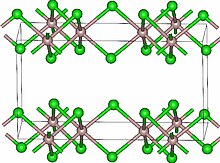
| |
| Names | |
|---|---|
| IUPAC name Lutetium(III) chloride | |
| Other names Lutetium chloride, lutetium trichloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.205 |
| PubChem CID | |
| RTECS number |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | LuCl3 |
| Molar mass | 281.325 g/mol |
| Appearance | colorless or white monoclinic crystals |
| Density | 3.98 g/cm³ |
| Melting point | 905°C |
| Boiling point | sublimes above 750°C |
| Solubility in water | soluble |
| Structure | |
| Crystal structure | Monoclinic, mS16 |
| Space group | C2/m, No. 12 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Xi (Irritant) |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Other anions | Lutetium(III) oxide |
| Other cations | Scandium(III) chloride Yttrium(III) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals. Lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.
Reactions
Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium:
References
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, p. 472, ISBN 0849305942, retrieved 2008-06-27
- "Chemistry: Periodic Table: Lutetium: compound data (lutetium (III) chloride)". WebElements. Retrieved 2008-06-27.
- Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 232, ISBN 0849386713, retrieved 2008-06-27
- "450960 Lutetium(III) chloride anhydrous, powder, 99.99% trace metals basis". Sigma-Aldrich. Retrieved 2008-06-27.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Patnaik, Pradyot (2004), Handbook of Inorganic Chemicals, Amsterdam: McGraw-Hill Professional, p. 244, ISBN 0070494398, retrieved 2008-06-27
| Lutetium compounds | |
|---|---|
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |